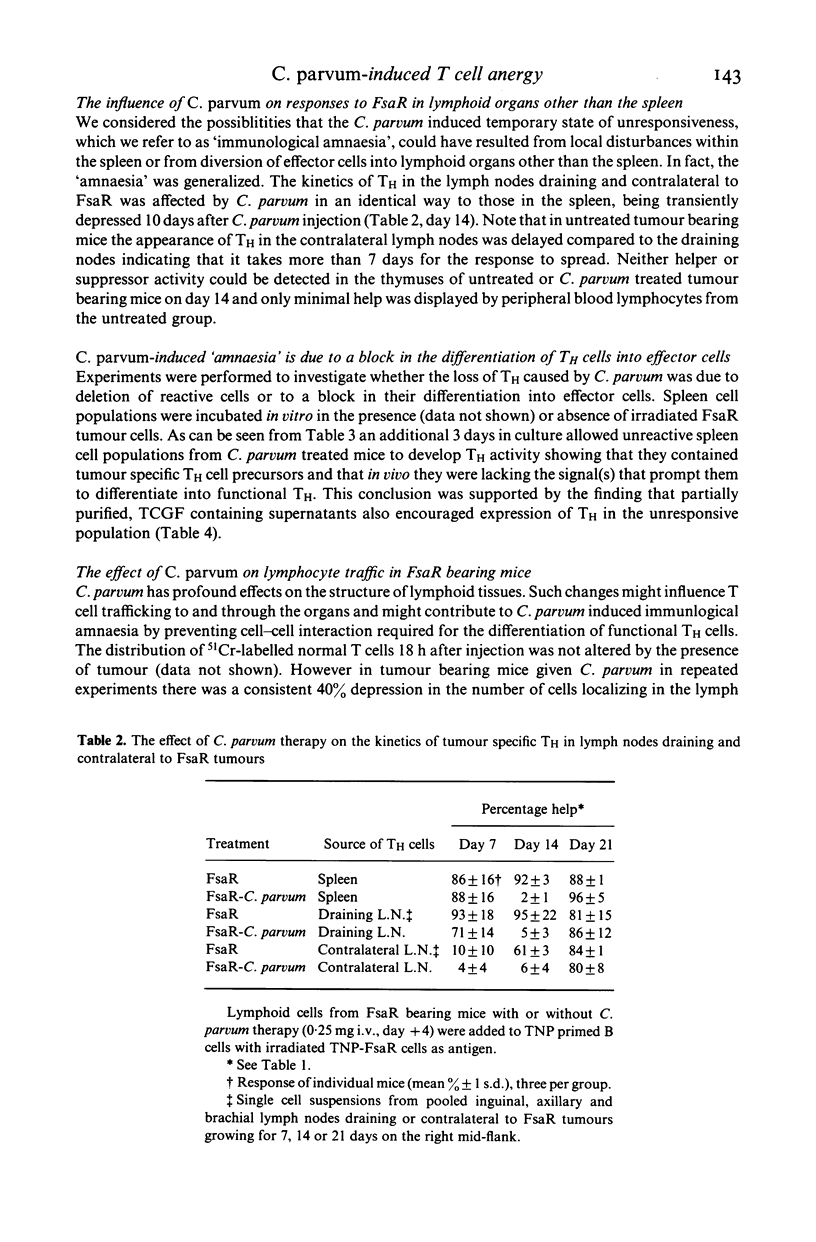
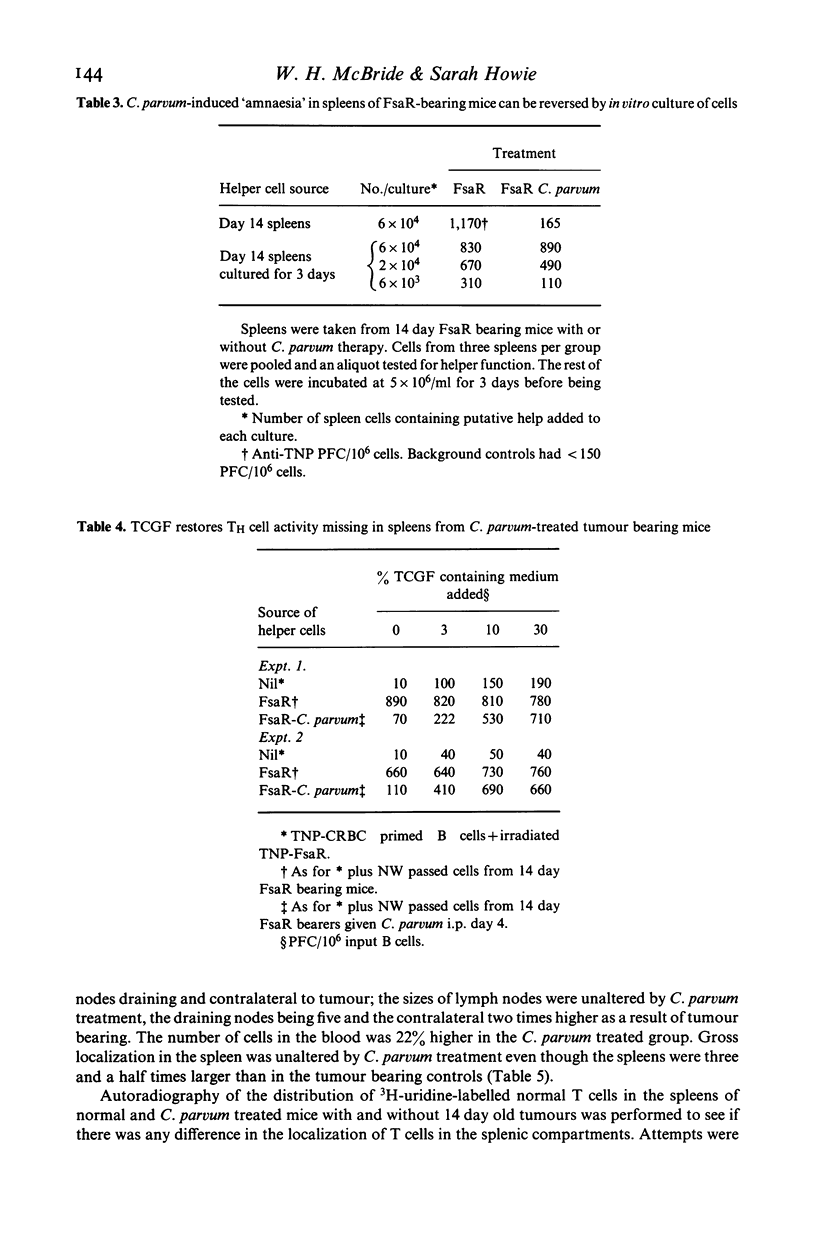
Abstract
Systemic administration of Corynebacterium parvum causes T cell-dependent regression of an established methylcholanthrene-induced murine fibrosarcoma beginning 10 days after Cp injection. At this time, tumour specific effector T cell responses measured by reactivity in a T helper cell assay or in a Winn assay disappear only to return later. We refer to this temporary lapse in T cell reactivity as immunological 'amnaesia'. Antigen specific T cell responses within all lymphoid organs appear to be affected. The 'amnaesic' state is characterised by the presence of primed T cells but the absence of T effector cells and suppressor cells. The differentiation of the primed T cells is blocked probably as a result on the non-delivery of a differentiation signal. There are several possible mechanisms which could account for this; the one we prefer is that cells are prevented from entering T cell-dependent cell interaction areas within lymphoid organs. This state of T cell 'amnaesia' may underlie anergy in some inflammatory, infectious and neoplastic diseases. The apparent paradox of T cell-dependent tumour regression occurring in mice with depressed T cell responses is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullock W. E. Anergy and infection. Adv Intern Med. 1976;21:149–173. [PubMed] [Google Scholar]
- Castro J. E. The effect of Corynebacterium parvum on the structure and function of the lymphoid system in mice. Eur J Cancer. 1974 Feb;10(2):115–120. doi: 10.1016/0014-2964(74)90062-0. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Greager J. A., Baldwin R. W. Influence of immunotherapeutic agents on the progression of spontaneously arising, metastasizing rat mammary adenocarcinomas of varying immunogenicities. Cancer Res. 1978 Jan;38(1):69–73. [PubMed] [Google Scholar]
- Günther J., Haas W., Von Boehmer H. Suppression of T cell responses through competition for T cell growth factor (interleukin 2). Eur J Immunol. 1982 Mar;12(3):247–249. doi: 10.1002/eji.1830120315. [DOI] [PubMed] [Google Scholar]
- Howie S., McBride W. H., James K. Distribution of IgM, IgA and IgG secreting cells in the tissues of normal and tumour-bearing mice. Immunology. 1982 May;46(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- Howie S., McBride W. H. Tumor-specific T helper activity can be abrogated by two distinct suppressor cell mechanisms. Eur J Immunol. 1982 Aug;12(8):671–675. doi: 10.1002/eji.1830120809. [DOI] [PubMed] [Google Scholar]
- James K., Woodruff M. F., McBride W. H., Willmott N. Serological changes associated with C. parvum treatment in nude mice. Br J Cancer. 1977 May;35(5):684–686. doi: 10.1038/bjc.1977.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Keller R. Abrogation of antitumor effects of Corynebacterium parvum and BCG by antimacrophage agents: brief communication. J Natl Cancer Inst. 1977 Dec;59(6):1751–1753. doi: 10.1093/jnci/59.6.1751. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Kumar V., Luevano E., Bennett M. Hybrid resistance to EL-4 lymphoma cells. I. Characterization of natural killer cells that lyse EL-4 cells and their distinction from marrow-dependent natural killer cells. J Exp Med. 1979 Sep 19;150(3):531–547. doi: 10.1084/jem.150.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride W. H., Dawes J., Dunbar N., Ghaffar A., Woodruff M. F. A comparative study of anaerobic Coryneforms. Attempts to correlate their anti-tumour activity with their serological properties and ability to stimulate the lymphoreticular system. Immunology. 1975 Jan;28(1):49–58. [PMC free article] [PubMed] [Google Scholar]
- Milas L., Hunter N., Basić I., Withers H. R. Complete regressions of an established murine fibrosarcoma induced by systemic application of Corynebacterium granulosum. Cancer Res. 1974 Oct;34(10):2470–2475. [PubMed] [Google Scholar]
- Milas L., Kogelnik H. D., Basic I., Mason K., Hunter N., Withers H. R. Combination of C. parvum and specific immunization against artificial pulmonary metastases in mice. Int J Cancer. 1975 Nov 15;16(5):738–746. doi: 10.1002/ijc.2910160506. [DOI] [PubMed] [Google Scholar]
- Moore K., McBride W. H. Enhanced Fc receptor expression by a sub-population of murine intra-tumour macrophages following intravenous Corynebacterium parvum therapy. Br J Cancer. 1983 Jun;47(6):797–802. doi: 10.1038/bjc.1983.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., McBride W. H. The activation state of macrophage subpopulations from a murine fibrosarcoma. Int J Cancer. 1980 Nov 15;26(5):609–615. doi: 10.1002/ijc.2910260513. [DOI] [PubMed] [Google Scholar]
- Peters L. J., McBride W. H., Mason K. A., Milas L. A role for T lymphocytes in tumour inhibition and enhancement caused by systemic administration of Corynebacterium parvum. J Reticuloendothel Soc. 1978 Jul;24(1):9–18. [PubMed] [Google Scholar]
- Scott M. T. Corynebacterium parvum as a therapeutic antitumor agent in mice. I. Systemic effects from intravenous injection. J Natl Cancer Inst. 1974 Sep;53(3):855–860. doi: 10.1093/jnci/53.3.855. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Corynebacterium parvum as a therapeutic antitumor agent in mice. II. Local injection. J Natl Cancer Inst. 1974 Sep;53(3):861–865. doi: 10.1093/jnci/53.3.861. [DOI] [PubMed] [Google Scholar]
- Toujas L., Dazord L., Martin A., Guelfi J. Simultaneous stimulation of plaque forming cells and depression of cellular immunity to sheep erythrocytes, after adjuvant treatment. Biomedicine. 1973 Nov 20;19(11):503–505. [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff M., Dunbar N., Ghaffar A. The growth of tumours in T-cell deprived mice and their response to treatment with Corynebacterium parvum. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):97–102. doi: 10.1098/rspb.1973.0034. [DOI] [PubMed] [Google Scholar]
- Zola H. Mitogenicity of Corynebacterium parvum for mouse lymphocytes. Clin Exp Immunol. 1975 Dec;22(3):514–521. [PMC free article] [PubMed] [Google Scholar]


