Abstract
The optimal vector, regulatory sequences, and method of delivery of angiogenic gene therapy are of considerable interest. The Spalax ehrenbergi superspecies live in subterranean burrows at low oxygen tensions and its tissues are highly vascularized. We tested whether continuous perimuscular administration of Spalax vascular endothelial growth factor (VEGF) DNA could increase tissue perfusion in a murine hindlimb ischemia model. Placebo or VEGF ± internal ribosome entry site (IRES) was continuously administrated perimuscularly in the ischemic zone by using an infusion pump. None of the mice in the VEGF-treated group (>50 μg) developed visible necrosis vs. 33% of the placebo group. Microscopic necrosis was observed only in the placebo group. Spalax VEGF muscular infiltration resulted in a faster and more complete restoration of blood flow. The restoration of blood flow by VEGF was dose-dependent and more robust and rapid when using the VEGF–IRES elements. The flow restoration using continuous perimuscular infiltration was faster than single i.m. injections. Vessel density was higher in the VEGF and VEGF–IRES (−) groups compared with the placebo. Continuous perimuscular administration of angiogenic gene therapy offers a new approach to restore blood flow to an ischemic limb. Incorporation of an IRES element may assist in the expression of transgenes delivered to ischemic tissues. Further studies are needed to determine whether VEGF from the subterranean mole rat Spalax VEGF is superior to VEGF from other species. If so, 40 million years of Spalax evolution underground, including adaptive hypoxia tolerance, may prove important to human angiogenic gene therapy.
Angiogenic gene therapy has been demonstrated to be a viable approach to promote blood flow to ischemic tissues in several animal models and recently in man (1–3). Considerable interest exists in the nature of the vector, regulatory sequences, and mode of delivery of these angiogenic agents (4–6). Vascular endothelial growth factor (VEGF), a potent angiogenic growth factor, has been used extensively in these models of angiogenic therapy by using either naked plasmid DNA or a viral vector delivered by a single or multiple i.m. or intraarterial injection to the ischemic region (7, 8).
Tissue ischemia results in an inhibition of the translation of the majority of cellular mRNAs, which depend on cap-dependent ribosomal scanning. However, a small subset of mRNAs, including that for VEGF, contains a bypass mechanism in its 5′ UTR termed an internal ribosome entry site (IRES) that permits the RNA to be efficiently translated under ischemic conditions (9, 10). Although the endogenous 1-kb 5′ UTR of VEGF contains two such IRES elements, these have not been used previously in attempts to augment VEGF expression in ischemic tissues.
In a mouse unilateral ischemic hindlimb model, we demonstrate the efficacy of a method of continuous perimuscular delivery of an angiogenic agent and the importance of the VEGF–IRES element in augmenting collateral formation and tissue perfusion. The VEGF used for this study is from the mole rat species Spalax judaei belonging to the superspecies Spalax ehrenbergi (11, 12). These rodents live in subterranean burrows at decidedly low oxygen tensions and have highly vascularized tissues (13, 14).
Methods
Mouse Hindlimb Ischemia: Animal Surgery.
All procedures were approved by the Animal Board and Safety Committee of the Faculty of Medicine, Technion–Israel Institute of Technology, and comply with all safety and regulatory guidelines. C57 mice, 10–12 weeks old, were anesthetized by i.p. pentobarbital injection (160 mg/kg) for surgical procedures and for subsequent laser Doppler analysis of hindlimb blood flow. The surgical procedure was performed under a microscope. Skin incisions were performed at the mid portion of the left hindlimb overlying the iliac artery. The iliac artery was then ligated proximally and distally with 4-0 silk ligatures and excised. All accessory arteries were ligated and cut. Before closure, a 200-μl osmotic infusion pump, measuring 20 mm (Alzet osmotic pumps, Durect, Cupertino, CA) and containing a specified concentration of the agent tested, was implanted intraabdominally with an outlet tube (25 G) from the pump fenestrated and tunneled into the left quadriceps muscle. The pump rate has been documented to be 1 μl/h, which given a 200-μl reservoir would allow continuous perimuscular pumping for ≈7 days. A drug of 500 μg results in a concentration of 2.5 μg/μl or 2.5 μg/h for 7 days. The overlying skin was then closed with continuous surgical sutures by using 3-0 silk ligatures. The surgery, hindlimb blood flow measurements, and pathological analysis were performed blindly without any knowledge of the treatment given.
Study Groups.
In the continuous infiltration arm using the osmotic pump we compared the VEGF plasmid (with IRES, 50–500 μg, n = 24), VEGF–IRES (−) plasmid (500 μg, n = 10), and a placebo group that was either saline placebo (n = 15) or the empty pTRE vector (n = 7) without any VEGF.
In a subset of animals, instead of inserting an osmotic pump, the VEGF vector (with IRES, 500 μg, n = 10) or placebo (n = 10) were given in four i.m. injections. The i.m. injections were given in four constant and identical places in the left quadriceps muscle of each animal tested in this group, at the time of the operation. The i.m. injections (each 50 μl; 2.5 μg/μl VEGF plasmid, same concentration as used with the osmotic pump) were done with a 27-gauge blunt needle.
Mole Rat VEGF.
The VEGF used in this study (accession no. AJ544164) was cloned from the blind subterranean mole rats of the superspecies S. ehrenbergi, which consists of 12 allospecies in the East Mediterranean region with four species in Israel (11, 12). The coding sequence of the three species of mole rat VEGF was described (15). The entire ≈1 kb of 5′ UTR of mole rat VEGF mRNA was cloned by RT-PCR and juxtaposed to the mole rat VEGF165 amino acid isoform (VEGF-165) coding sequence. The sequence homology of mole rat, rat, mouse, and human VEGF is >90% in the coding region (15). In the 5′ UTR of VEGF, the homology is similarly high in the region of the 5′ UTRs that have been identified in human VEGF mRNA to encode two distinct IRES elements (A.A. and E.N., unpublished data).
Vector/Regulatory Sequences Used.
We used the cDNA for VEGF-165 with its 1 kb of 5′ UTR of the species S. judaei (2n = 60; Anza, Shomron, Israel) belonging to the superspecies S. ehrenbergi (11). The cDNA was cloned into the pTRE plasmid (CLONTECH) hereafter referred to as the VEGF vector. This vector used the 763-bp cytomegalovirus promoter/enhancer element to drive VEGF transcription. Downstream of the VEGF cDNA sequences there was an intron and a polyadenylation signal sequence derived from SV 40. The vector was purified by using Qiagen (Hilden, Germany) columns and resuspended in water at the desired concentration. For studies without the IRES [VEGF–IRES (−) vector] the entire 5′ UTR (except for 20 bp of 5′ to the initiation codon) was deleted from the VEGF pTRE plasmid described previously. Placebo animals received only normal saline in the osmotic pump. A second “placebo” group received only the backbone pTRE vector without the VEGF gene.
Monitoring Hindlimb Blood Flow.
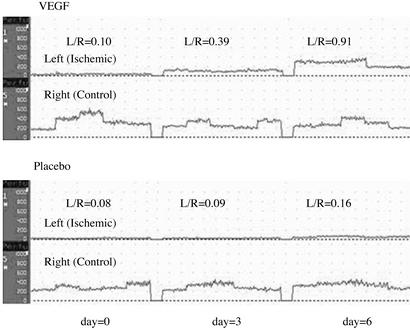
Ischemic (left)/normal (right) limb blood flow ratio was measured by using a laser Doppler blood flow meter (Perimed 4000, Perimed, Stockholm). Measurements were performed by multichannel laser Doppler flow meter (PeriFlux 4001 Master, Perimed) at a wavelength of 780 nm, using two probes (PF 415:42) with fiber separation of 0.5 mm. For measurement of capillary perfusion, the probes were placed perpendicular to the foot. Recordings were obtained continuously for 2 min each at five different points over the mouse foot. The capillary blood flow was calculated with perisoft software (Version 5.10C2) by multiplying the concentration of moving blood cells (concentration units) by mean velocity of the cells (velocity units) and expressed as perfusion units (ref. 16; Fig. 1).
Figure 1.
Ischemic (left)/normal (right) (L/R) limb blood flow ratio. Measurements were done by using a laser Doppler blood flow meter. The capillary blood flow was expressed as perfusion units (concentration of moving blood cells multiplied by the mean velocity of the cells). The mean values were obtained from five different recording points on the mouse foot. Hindlimb blood flow was monitored every 3 days. (Upper) Time course of the ratio of the flow (L/R) in a single representative VEGF-treated mouse. (Lower) Time course of the ratio of the flow in a single representative mouse treated only with normal saline.
The laser Doppler uses a 12-mW helium neon laser beam that sequentially scans a defined surface area. A photodiode in the scanner collects the backscattered light emitted by the laser, and the shift in the frequency of the incident light is transformed into voltage variations in the range of 0–10 V, which is directly related to blood flow distribution. Studies have validated that Doppler flow velocity correlates with capillary density in the ischemic limb (17). Hindlimb blood flow was monitored every 3 days until reaching a plateau or a normal value.
Immunohistochemistry.
Animals were killed at predetermined time points after surgery by an overdose of sodium pentobarbital. For each limb, three proximal areas (quadriceps muscle) and one distal area (gastrocnemius muscle) were analyzed.
For immunohistochemical analysis, 5-μm-thick sections were prepared from paraffin-embedded tissue samples of the limbs. Sections were cut with the muscle fibers oriented transversely. Identification of endothelial cells was performed by immunostaining for factor VIII-related antigen by using a polyclonal anti-factor VIII antiserum at a 1/20 dilution (Zymed). Immunostaining for smooth muscle actin by using a mouse mAb at 1/100 dilution (Zymed) was used to identify smooth muscle cells. Immunohistochemistry was performed on an automated system (Ventanu Medical Systems, Tucson, AZ). Capillary density in both ischemic and nonischemic limbs was determined by counting endothelial cells positively stained with factor VIII under light microscopy. Analysis of smooth muscle cells was performed to differentiate arterioles from capillaries or venules. The microvessel density (obtained by either factor VIII or smooth muscle actin staining) was computed by using a computerized image analysis system composed of a trichip RGB video camera (Sony, Tokyo) installed on a light microscope (Zeiss) and attached to a personal computer equipped with a frame grabber. Histological images were captured, digitized, and displayed on a high-resolution monitor. Five microscopic fields were analyzed by using a medium-sized magnifying lens (×200) and were loaded on a 760 × 570-pixel buffer area. The microvessel density parameter was measured by using IMAGE PRO-PLUS-4 image analysis software (Media Cybernetics, Silver Spring, MD).
Thirty randomly chosen high-power fields in two different light microscopic sections from the four sites in each limb of each animal were counted, and blood vessel density was expressed as number of blood vessels per mm2. A ratio in each level, between the legs, was then calculated.
Detection of Transgene in Vivo.
Tissue localization of mole rat VEGF DNA that was infused was determined by PCR by using mole rat-specific primers (sense primer 5′-CGGAATTCGAAGTTTATGGACGTCTTCCG-3′ and antisense primer 5′-CGGGATCCTGGTCTGCATTCACATCTGTTG-3′). The PCR conditions used for this assay were 94°C for 5 min followed by 55°C for 1 min, 72°C for 1 min, and 94°C for 45 sec for 35 cycles.
Statistical Analysis.
Multiple parametric groups were compared by using the one-way ANOVA test followed by the Bonferroni post hoc test. P values of 0.05 or less were considered to be statistically significant.
Results
Necrosis.
Immediately after the operation the operated limb developed a blue-gray color. Some of the mice developed grossly visible necrosis and autoamputation of the ischemic limb. None of the mice treated with >50 μg of VEGF developed necrosis as compared with 33% of the mice in the placebo group (P < 0.05). The placebo group includes both the saline as well as pTRE-injected groups; no difference was noted between them in necrosis ratio or flow rate restoration. Only 2/20 mice developed necrosis in the i.m. injection, 1 in the VEGF and 1 in the placebo treatment group.
Restoration of Flow.
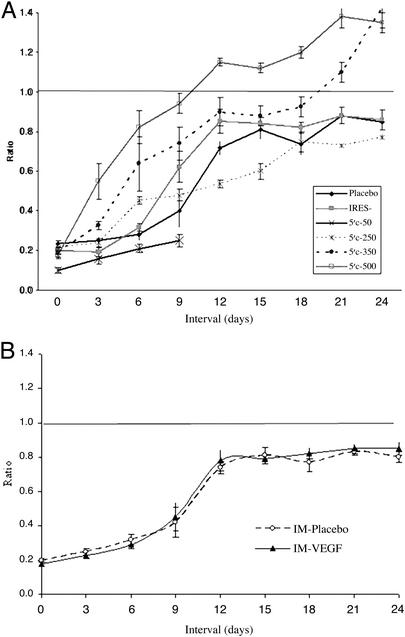
Preoperatively, the ratio of blood flow between the two hindlimbs for all animals used in this study was 1. No differences were found in the time course of restoration of blood flow using a saline placebo or an empty pTRE vector without any VEGF. Therefore, these two placebo groups, both involving continuous infusion, were pooled together. Animals which developed necrosis were not included in the flow measurements because of gross tissue loss (autoamputation), which precluded measurement of blood flow. In those mice in the placebo group that did not undergo autoamputation, flow returned to normal only after 21 days (Fig. 2A). A more complete and rapid restoration of blood flow was observed in the VEGF-treated mice. Differences in the flow between the placebo and VEGF-treated groups became statistically significant by day 6 (Fig. 2A). The VEGF–IRES (−) group restored its flow significantly faster than the placebo group but slower (P < 0.05 day 6) than the VEGF group. The plateau in the ratio of the flow between the ischemic and nonischemic limb, 21 days after surgery, was also different among the groups. In the animals treated with VEGF the blood flow in the limb with the infusion pump eventually surpassed that in the normal limb (ratio of >1.0 at 21 days); however, in the VEGF–IRES (−) and placebo groups, the flow in the limb with the infusion pump did not reach levels obtained in the normal limb (ratio of only 0.8–0.9 at 21 days). Placebo animals that were maintained for up to 35 days still failed to restore their flow ratio to 1.0.
Figure 2.
(A) Time-dependent changes in flow. Restoration of blood flow in the ischemic limb in VEGF-, VEGF-IRES (−)-, and placebo-treated animals and according to VEGF dosage. The time-dependent change in ratio of flow between the ischemic and nonischemic limb for different dosages of the VEGF vector placed in the infusion pump in an identical volume. A significantly faster restoration of blood flow was achieved with the VEGF vectors as compared with a placebo, and with the VEGF–IRES (+) vector compared with the VEGF–IRES (−) vector. Restoration of flow was more complete (achieving a ratio of flow between the two limbs of at least 1.0) in the VEGF group than in the VEGF–IRES (−) or placebo groups. Preoperatively, the ratio of blood flow for all animals used in this study was 1. Day 0 indicates the ratio of flow immediately after surgery. A minimum of four animals was used for each VEGF dosage group and each time point. Data are presented as mean ± SEM. (B) Restoration of blood flow in the ischemic limb in for the i.m. injection route of delivery. There are no statistically significant differences between the VEGF–IRES (+) and placebo arms. The VEGF vector (500 μg/200 μl) or saline placebo (200 μl) was given in four i.m. injections (each of 50 μl) by using a 27-gauge blunt needle. A minimum of four animals was used for each group and each time point.
There was a dose-dependent effect with a more rapid restoration of flow with the higher dosages of the VEGF vector. By day 6 the blood flow was almost complete for the group treated with 350 and 500 μg of VEGF. Application of 50 μg of VEGF was not sufficient to induce any beneficial effect on restoration of blood flow.
Restoration of blood flow with 500 μg of VEGF vector administered i.m. was slower than with the infusion pump. The i.m. group reached an average ratio of 0.85, with no statistically significant differences compared with a placebo (Fig. 2B).
Vessel Density Ratio.
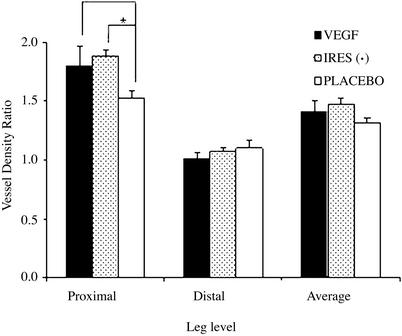
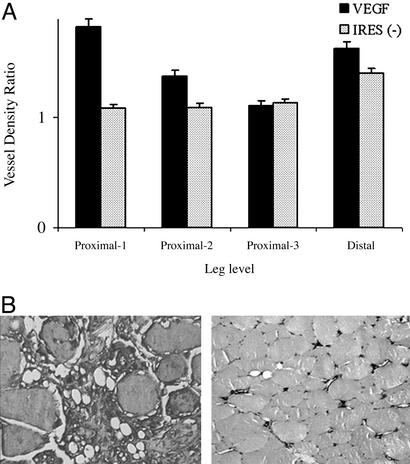
In this study we used immunohistochemical methods permitting identification of factor VIII-related antigen to detect endothelial cells representing capillaries as well as arterioles, and smooth muscle actin to identify blood vessels containing a smooth muscle cell coat (i.e., arterioles). In this fashion we sought to attempt to differentiate between angiogenesis and arteriogenesis. Because all blood vessels come from preexisting blood vessels (angiogenesis) and then mature from capillaries into larger vessels by the process of arteriogenesis, an increase in the total number of endothelial cells was interpreted as being indicative of angiogenesis whereas an increase in the number of smooth muscle cells was interpreted as indicative of arteriogenesis. The blood vessel density ratio (ischemic to nonischemic limb) for smooth muscle actin is presented in Fig. 3 and for factor VIII in Fig. 4A. The endothelial cell density ratio could not be determined for the placebo group because of an excess of microscopic necrosis in the ischemic limb of these animals (Fig. 4B). The blood vessel density (as measured with factor VIII) was higher in the ischemic leg in both the proximal and distal portion examined, representing a higher number of capillaries not just in the area of VEGF infiltration but also distally in the region of most severe ischemia, suggesting that angiogenesis was occurring both proximally and distally. The increase in blood vessel density in the ischemic limb, as compared with the nonischemic limb as measured with actin, was statistically significantly higher for the VEGF and VEGF–IRES (−)-treated animals compared with the placebo only in the proximal region. No differences were observed in the distal portion, suggesting that arteriogenesis was occurring only in the proximal and not in the distal region. Importantly, no angioma formation was detected with this method of delivery.
Figure 3.
Vessel density ratio of smooth muscle actin. Vessel density based on actin staining was performed and quantitated as defined in Methods. The ratio of the vessel density between the ischemic and nonischemic limb in the proximal region was >1.0 in all three groups, suggesting that arteriogenesis was occurring in all groups in that region. The ratio of the vessel density between the ischemic and nonischemic limbs was significantly greater in the proximal region in the VEGF and IRES (−) animals as compared with the placebo group (P < 0.05), suggesting that VEGF increased arteriogenesis proximally. A minimum of three animals was used for the group.
Figure 4.
Factor VIII vessel density ratio. Vessel density based on factor VIII staining was performed and quantitated as defined in Methods. (A) The ischemic limb vessel density was significantly greater than that in the nonischemic limb in the proximal and distal regions, suggesting that angiogenesis was occurring both proximally and distally in these groups. Due to extensive microscopic necrosis it was not possible to quantitate factor VIII density in the placebo animals and therefore to compare VEGF- and placebo-treated animals for this parameter. A minimum of three animals was used for group. (B) Representative picture of the massive microscopic necrosis observed in the ischemic hindlimb of the placebo group (Left). No microscopic necrosis was observed in the VEGF/VEGF–IRES (−) groups (Right).
Isolation of the Vector.
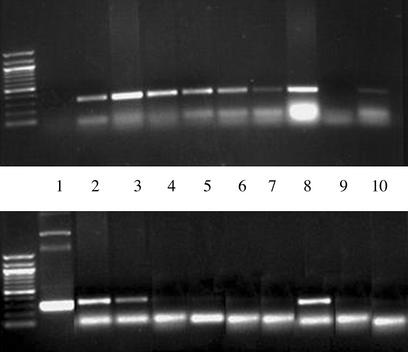
We determined the tissue localization of the DNA vector that was delivered by infusion pump 2 days after the pump had emptied by using PCR and primers specific for mole rat VEGF. The mole rat VEGF DNA was detected only in the proximal and distal portions of the ischemic leg and in the liver (Fig. 5) but not in the normal leg or in other tissues.
Figure 5.
Tissue localization of mole rat VEGF. DNA from various tissues was prepared as described in Methods. The presence or absence of mole rat DNA was ascertained by PCR with mole rat-specific primers, days 9–11 after operation. This procedure was repeated in three animals. (Upper) Presence of a specific band detecting 18S murine DNA sequences by PCR documenting the presence of DNA in each sample. (Lower) Presence or absence of mole rat VEGF in different tissues. Lanes: 1, control vector; 2, left (ischemic) lower proximal limb; 3, left lower distal limb; 4, right (control) lower proximal limb; 5, right lower limb; 6, left upper limb; 7, right upper limb; 8, liver; 9, negative control; 10, heart. Mole rat sequences were detected only in the proximal and distal portions of the ischemic limb and from the liver.
Discussion
In this study we demonstrate in a murine hindlimb ischemia model rapid and robust restoration of blood flow and complete tissue salvage by using naked plasmid DNA encoding VEGF of the subterranean mole rat species S. judaei of the S. ehrenbergi superspecies (11) in a method for gene delivery, continuous perimuscular infiltration. This difference in the restoration of blood flow rates between the VEGF-treated and placebo groups may underestimate the real difference in the outcome of animals from these two groups because approximately one-third of the placebo group developed gross necrosis, and these animals were excluded from all flow determinations. Moreover, we have shown that when using plasmid DNA, continuous administration is superior to multiple simultaneous i.m. injections. Given the known low efficiency of uptake of injected naked plasmid DNA, continuous infusion may result in a higher transfection efficiency than single or repeated injections. Furthermore, prolonged and continuous exposure to angiogenic growth factors may be critical in obtaining an optimal angiogenic response (7, 18).
We have also demonstrated that an IRES element is functionally important for the biological activity of a molecule in vivo. The two IRES elements in the VEGF 5′ UTR (9) allow for the efficient translation of VEGF mRNA under ischemic conditions. In the absence of these IRES elements, VEGF mRNA, similar to most cellular RNA lacking IRES elements, is not translated efficiently under hypoxic conditions due to a limitation in critical components of the cap-dependent protein translation initiation process, notably eIF4. In this study, the restoration of blood flow was slower and less complete for the vector without the IRES elements than for the VEGF vector with the IRES. These findings are consistent with the requirement that the translational efficiency of VEGF mRNA in ischemic tissues be maintained and not adversely affected by an insult that impairs the generalized cellular translational apparatus.
In this study, we used the VEGF isolated from the mole rat S. judaei belonging to the superspecies S. ehrenbergi (2n = 60; ref. 11). Although it remains to be demonstrated that mole rat VEGF has a different potency or spectrum of activity from human, rat, or murine VEGF, it is clear that the vasculature in general and the regulation of VEGF in particular in the mole rat is unique (11–15). The entire life cycle of the mole rat is spent underground in subterranean burrows at decidedly low oxygen tensions. The critical pO2 (the lowest oxygen pressure supporting life) is lower for the mole rat than for any other mammal studied to date (2, 13, 19).
This capacity to withstand hypoxic conditions even exceeds that manifested by many high-altitude and diving mammals. Since its origin some 40 million years ago, Spalax has evolved adaptations to life underground that allow it to survive and carry out normal activities in a highly hypoxic environment (14, 15). The muscular tissues of Spalax harbor a larger capillary density (+31%) than the rat, resulting in a reduced diffusion distance to mitochondria (14). This increase in vascular density was found to be associated with up-regulated expression of VEGF even under normoxic conditions (15), suggesting that the regulation of VEGF in the mole rat may be different from that in other species. The structural pulmonary diffusing capacity for O2 was greater in the mole rat (+44%), thus facilitating O2 uptake in hypoxia. Thus, structural adaptations in lung and muscle tissues improve O2 diffusion conditions and serve to maintain high metabolic rates in hypoxia but have no consequences for achieving V O2 max under normoxic conditions (14). The tissues of Spalax have been shown to be highly vascularized, resulting in a shorter diffusion distance for oxygen.
In this study, young and old mice that have an impaired ability to spontaneously collateralize (25) were not examined. This study has not compared angiographic data among the groups; however, the interpretation is technically difficult and limited. Although a prolonged continuous exposure to angiogenic growth factors may be critical to obtaining an optimal angiogenic response, there are theoretical potential deleterious effects of continuous exposure to angiogenic growth factors including angioma formation.
The importance of angiogenesis, arteriogenesis, and vasodilation to the observed increase in blood flow with angiogenic therapy is a matter of considerable debate and controversy (20–22). Although it is clear that angiogenic therapy can induce angiogenesis, it has been argued as implausible, based on physical principles, that an increase in capillary vessels impacts substantially on blood flow (1). Nonetheless, VEGF, which is angiogenic and not arteriogenic, has been demonstrated to increase flow in numerous animal models and by multiple investigators (22–27). Furthermore, neutralizing antiserum to VEGF prevents the normal restoration of blood flow seen in the natural adaptation to ischemia (28, 29). We have demonstrated here that angiogenesis as well as arteriogenesis is occurring in mice treated with VEGF. The lack of a clear correlation between restoration of limb flow and capillary vessel density in our model would seem to indicate that angiogenesis is not primarily responsible for the return of blood flow. Furthermore, we cannot rule out a significant affect mediating the return of blood flow mediated by VEGF-augmented vasodilation. These questions may ultimately be answered more directly in murine models, such as that described here, by using targeted disruption and overexpression of genes involved in these distinct processes.
The evolution of blind subterranean mole rats, Spalacidae, started some 40 million years ago in Upper Eocene times (13). Their adaptive strategies at all molecular and organismal levels are uniquely adapted to underground challenges of darkness, hypoxia, hypercapnia, low productivity, and resource competition (14). Their respiratory adaptations, partly shared with other subterranean mammals across the planet but climaxed in spalacids, seem to have evolved a genetic battery that can effectively cope with hypoxic stresses (13). This molecular evolution machinery is now wide open to the use of human gene therapy.
Acknowledgments
We are grateful to Drs. Jonathan Leor and Andre Terzie for constructive comments that improved the paper. This work was supported by National Heart, Lung, and Blood Institute Grants RO1 HL-58510 and RO1 HL-58510, the Israel Cancer Research Fund, the Israel Cancer Association, the Bruce Rappaport Fund for Biomedical Research (all A.P.L.), the Israel Science Foundation (A.P.L., A.A., and A.H.), and the Ancell–Teicher Research Foundation for Molecular Genetics and Evolution (E.N.).
Abbreviations
- VEGF
vascular endothelial growth factor
- IRES
internal ribosome entry site
References
- 1.Schaper W, Ito W D. Circ Res. 1996;79:911–919. doi: 10.1161/01.res.79.5.911. [DOI] [PubMed] [Google Scholar]
- 2.Isner J M. J Clin Invest. 2000;106:615–619. doi: 10.1172/JCI10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond H K, McKirnan M D. Cardiovasc Res. 2001;49:561–567. doi: 10.1016/s0008-6363(00)00257-1. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Bonow R O, Chronos N A. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N. Kidney Intern. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 6.Kornowski R, Fuchs S, Leon M B, Epstein S E. Circulation. 2000;101:454–458. doi: 10.1161/01.cir.101.4.454. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 8.Henry T D. BMJ. 1999;318:1536–1539. doi: 10.1136/bmj.318.7197.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huez I, Creancier L, Audigier S, Gensac M C, Prats A C, Prats H. Mol Cell Biol. 1998;18:6178–6190. doi: 10.1128/mcb.18.11.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Mol Cell Biol. 1998;18:3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nevo E, Ivanitskaya E, Beiles A. Adaptive Radiation of Blind Subterranean Mole Rats: Naming and Revisiting the Four Sibling Species of the Spalax ehrenbergi Superspecies in Israel. Leiden, The Netherlands: Backhuys Publishers; 2001. [Google Scholar]
- 12.Nevo E. Evol Biol. 1991;25:1–125. [Google Scholar]
- 13.Nevo E. Mosaic Evolution of Subterranean Mammals: Regression, Progression and Global Convergence. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- 14.Widmer H P, Hoppeler H, Nevo E, Taylor C R, Weibel E W. Proc Natl Acad Sci USA. 1997;94:2062–2067. doi: 10.1073/pnas.94.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avivi A, Resnick M B, Nevo E, Joel A, Levy A P. FEBS Lett. 1999;452:133–140. doi: 10.1016/s0014-5793(99)00584-0. [DOI] [PubMed] [Google Scholar]
- 16.Rubinstein I, Abassi Z A, Coleman R, Milman F, Winaver J, Better O S. J Clin Invest. 1998;101:1325–1333. doi: 10.1172/JCI810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leahy M J, de Mul F F, Nilsson G E. Technol Health Care. 1999;7:143–162. [PubMed] [Google Scholar]
- 18.Edward M, Conway E M, Collen D, Carmeliet P. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 19.Arieli R, Arieli M, Heth G, Nevo E. Experientia. 1984;40:512–514. doi: 10.1007/BF01952413. [DOI] [PubMed] [Google Scholar]
- 20.van Royen N, Piek J J, Buschmann I, Hoefer I, Voskuil M, Schaper W. Cardiovasc Res. 2001;49:543–553. doi: 10.1016/s0008-6363(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 21.Buschmann I, Schaper W. J Pathol. 2000;190:338–342. doi: 10.1002/(SICI)1096-9896(200002)190:3<338::AID-PATH594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Risau W. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 23.Baffour R, Berman J, Garb J L, Rhee S W, Kaufman J, Friedmann P. J Vasc Surg. 1992;16:181–191. [PubMed] [Google Scholar]
- 24.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Cardiovasc Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 25.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner J M. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 26.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner J M. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita S, Zheng L P, Brogi E, Kearney M, Pu L Q, Bunting S, Ferrara N, Symes J F, Isner J M. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeshita S, Weir L, Chen D, Zheng L P, Riessen R, Bauters C, Symes J F, Ferrara N, Isner J M. Biochem Biophys Res Commun. 1996;227:628–635. doi: 10.1006/bbrc.1996.1556. [DOI] [PubMed] [Google Scholar]
- 29.Freedman S, Isner J M. J Mol Cell Cardiol. 2001;33:379–393. doi: 10.1006/jmcc.2000.1329. [DOI] [PubMed] [Google Scholar]