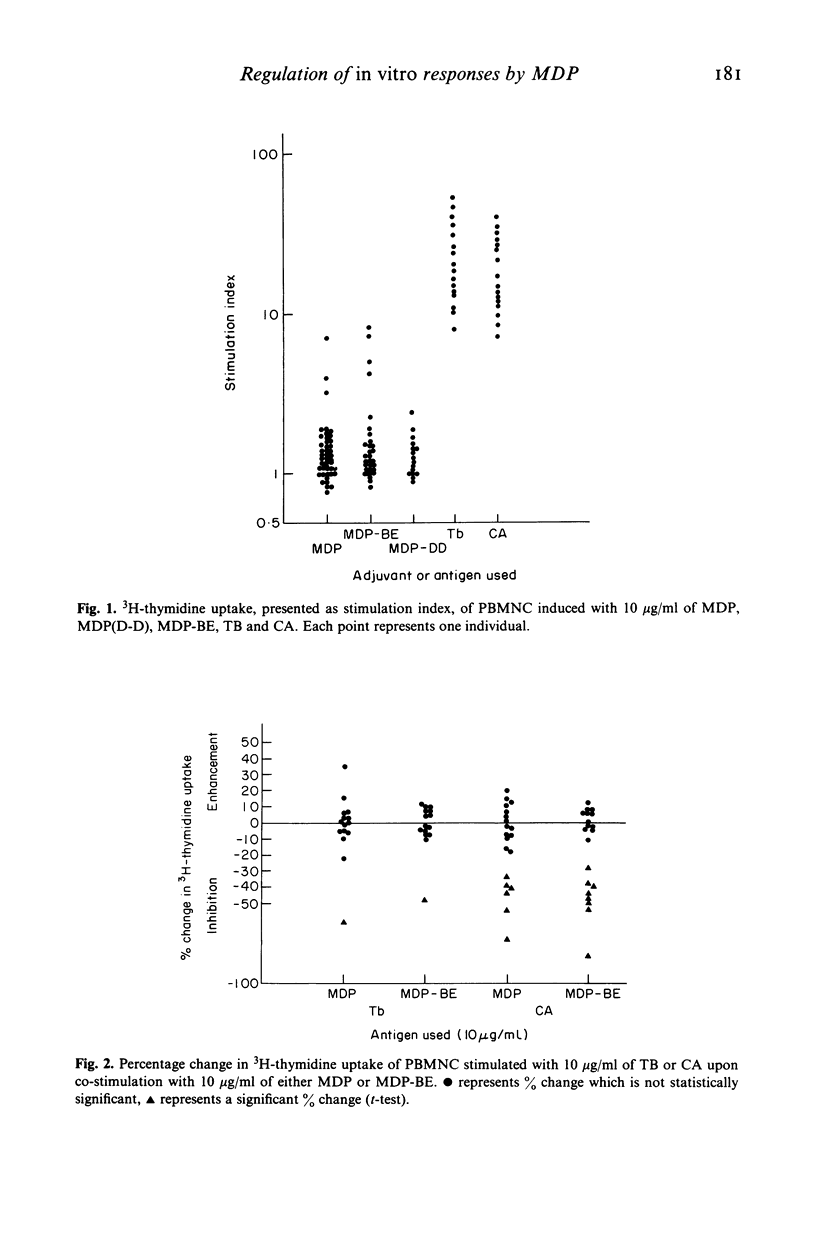
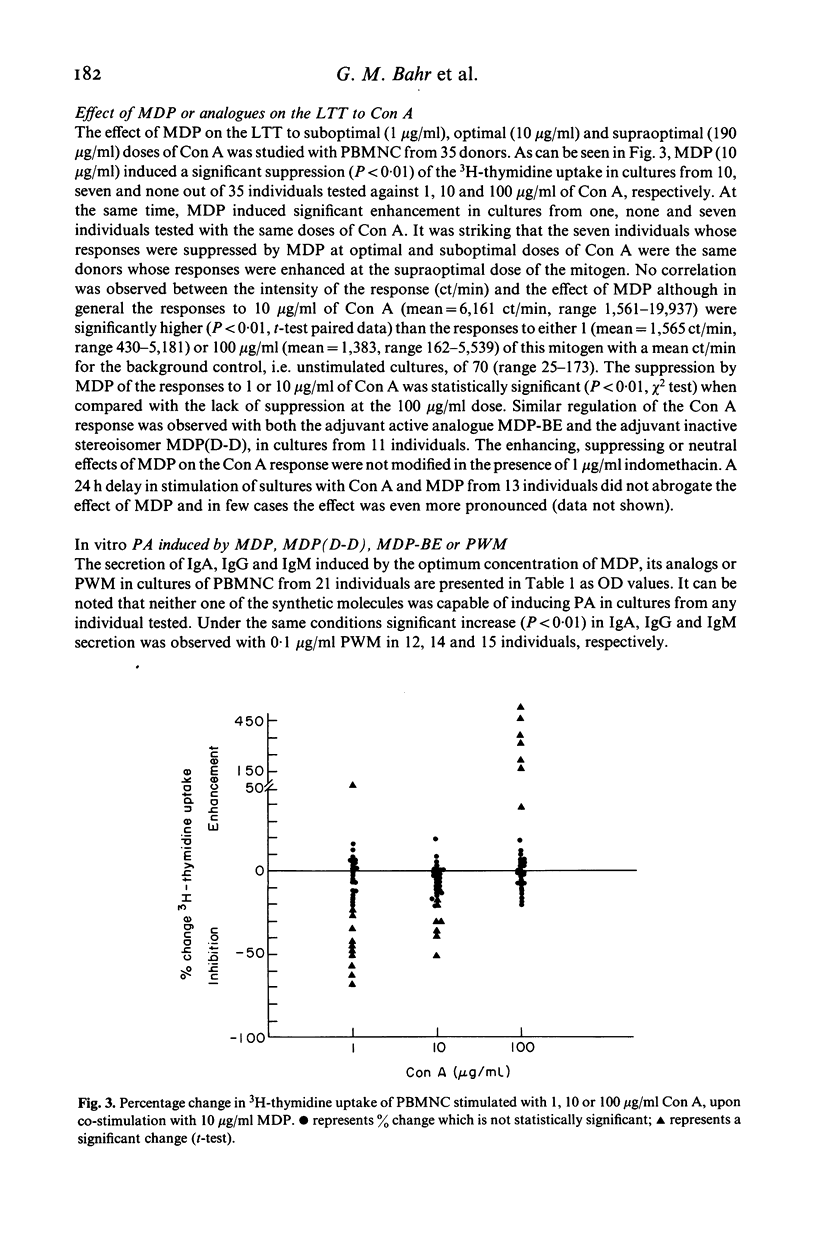
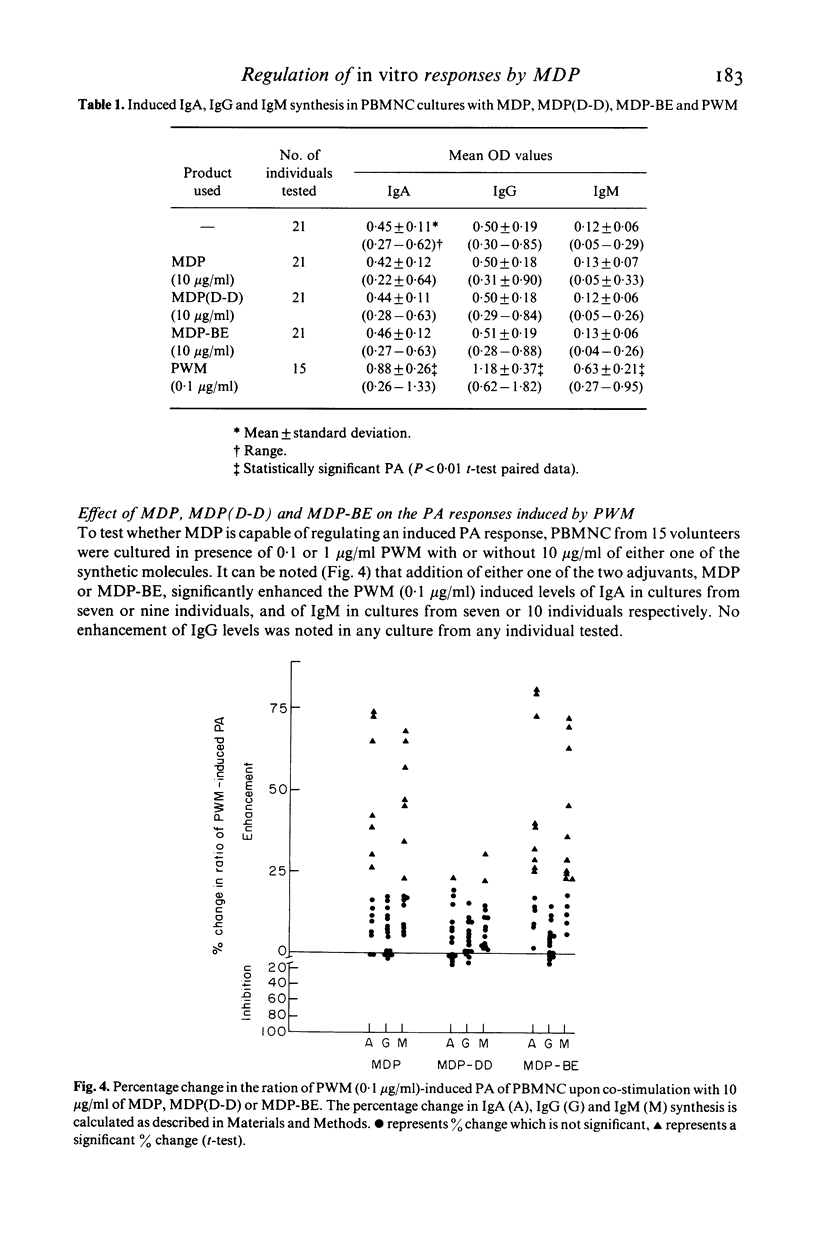
Abstract
The ability of muramyl dipeptide (MDP), its adjuvant inactive stereoisomer, MDP(D-D), and the non-pyrogenic, adjuvant active analogue, MDP-butyl ester (MDP-BE), to induce in vitro proliferation and/or polyclonal activation (PA) of peripheral blood mononuclear cells (PBMNC) from normal volunteers, was studied. MDP, as well as its two analogues, were incapable of inducing 3H-thymidine uptake or immunoglobulin synthesis in PBMNC cultures from the majority of the individuals tested. However, these muramyl peptides were capable of regulating the in vitro proliferative responses of some individuals to concanavalin A and to soluble antigens of Candida albicans. At the same time, enhancement of the pokeweed mitogen-induced IgA and IgM but not IgG PA was observed with MDP, its adjuvant active analogue MDP-BE, but not with the adjuvant inactive stereoisomer MDP(D-D). Results are discussed with relation to a possible genetic restriction of the responsiveness to MDP.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Audibert F., Jolivet M., Chedid L., Arnon R., Sela M. Successful immunization with a totally synthetic diphtheria vaccine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5042–5046. doi: 10.1073/pnas.79.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr G. M., Rook G. A., Stanford J. L. Inhibition of the proliferative response of peripheral blood lymphocytes to mycobacterial or fungal antigens by co-stimulation with antigens from various mycobacterial species. Immunology. 1981 Nov;44(3):593–598. [PMC free article] [PubMed] [Google Scholar]
- Carelli C., Audibert F., Gaillard J., Chedid L. Immunological castration of male mice by a totally synthetic vaccine administered in saline. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5392–5395. doi: 10.1073/pnas.79.17.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L. A., Parant M. A., Audibert F. M., Riveau G. J., Parant F. J., Lederer E., Choay J. P., Lefrancier P. L. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982 Feb;35(2):417–424. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Lefrancher P., Choay J., Lederer E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci U S A. 1977 May;74(5):2089–2093. doi: 10.1073/pnas.74.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Kunkel H. G. Induction of polyclonal antibody synthesis by human allogeneic and autologous helper factors. J Exp Med. 1979 Jun 1;149(6):1543–1548. doi: 10.1084/jem.149.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L., Lefrancier P., Choay J. In vitro spleen cell responsiveness to various analogs of MDP (N-acetylmuramyl-L-alanyl-D-isoglutamine), a synthetic immunoadjuvant, in MDP high-responder mice. Cell Immunol. 1978 Jan;35(1):173–179. doi: 10.1016/0008-8749(78)90137-5. [DOI] [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L. Nonspecific activation of murine spleen cells in vitro by a synthetic immunoadjuvant (N-acetyl-muramyl-L-alanyl-D-isoglutamine). Cell Immunol. 1977 Nov;34(1):49–56. doi: 10.1016/0008-8749(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Damais C., Riveau G., Parant M., Gerota J., Chedid L. Production of lymphocyte activating factor in the absence of endogenous pyrogen by rabbit or human leukocytes stimulated by a muramyl dipeptide derivative. Int J Immunopharmacol. 1982;4(5):451–462. doi: 10.1016/0192-0561(82)90020-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Elin R. J., Chedid L., Wolff S. M. The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. J Infect Dis. 1978 Dec;138(6):760–767. doi: 10.1093/infdis/138.6.760. [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Hilfiker M. L. Antigen-nonspecific helper factors in the antibody response. Fed Proc. 1982 Feb;41(2):263–268. [PubMed] [Google Scholar]
- Geha R. S., Mudawwar F., Schneeberger E. The specificity of T-cell helper factor in man. J Exp Med. 1977 Jun 1;145(6):1436–1448. doi: 10.1084/jem.145.6.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G. S., Rubin B., Sorensen S. F. Human leucocyte responses in vitro. I. Transformation of purified T lymphocytes with and without addition of partially purified monocytes. Clin Exp Immunol. 1977 Aug;29(2):295–303. [PMC free article] [PubMed] [Google Scholar]
- Juy D., Chedid L. Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4105–4109. doi: 10.1073/pnas.72.10.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc C., Juy D., Bourgeois E., Chedid L. In vivo regulation of humoral and cellular immune responses of mice by a synthetic adjuvant, N-acetyl-muramyl-L-alanyl-D-isoglutamine, muramyl dipeptide for MDP. Cell Immunol. 1979 Jun;45(1):199–206. doi: 10.1016/0008-8749(79)90377-0. [DOI] [PubMed] [Google Scholar]
- Lefrancier P., Choay J., Derrien M., Lederman I. Synthesis of N-acetyl-muramyl-L-alanyl-D-isoglutamine, an adjuvant of the immune response, and of some n-acetyl-muramyl-peptide analogs. Int J Pept Protein Res. 1977;9(4):249–257. doi: 10.1111/j.1399-3011.1977.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Lefrancier P., Derrien M., Jamet X., Choay J., Lederer E., Audibert F., Parant M., Parant F., Chedid L. Apyrogenic, adjuvant-active N-acetylmuramyl-dipeptides. J Med Chem. 1982 Jan;25(1):87–90. doi: 10.1021/jm00343a018. [DOI] [PubMed] [Google Scholar]
- Levitt D., Dagg M. K. Human B-lymphocyte subpopulations. II. Plasma cell differentiation of isotype-specific B lymphocytes from peripheral blood. Clin Immunol Immunopathol. 1981 Oct;21(1):50–61. doi: 10.1016/0090-1229(81)90194-x. [DOI] [PubMed] [Google Scholar]
- Löwy I., Leclerc C., Chedid L. Induction of antibodies directed against self and altered-self determinants by a synthetic adjuvant, muramyl dipeptide and some of its derivatives. Immunology. 1980 Mar;39(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Carswell J. W. Multiple skin testing in leprosy. J Hyg (Lond) 1975 Aug;75(1):57–68. doi: 10.1017/s0022172400047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Specter S., Cimprich R., Friedman H., Chedid L. Stimulation of an enhanced in vitro immune response by a synthetic adjuvant, muramyl dipeptide. J Immunol. 1978 Feb;120(2):487–491. [PubMed] [Google Scholar]
- Specter S., Friedman H., Chedid L. Dissociation between the adjuvant vs mitogenic activity of a synthetic muramyl dipeptide for murine splenocytes. Proc Soc Exp Biol Med. 1977 Jul;155(3):349–352. doi: 10.3181/00379727-155-39804. [DOI] [PubMed] [Google Scholar]
- Staruch M. J., Wood D. D. Genetic influences on the adjuvanticity of muramyl dipeptide in vivo. J Immunol. 1982 Jan;128(1):155–160. [PubMed] [Google Scholar]
- Wood D. D., Staruch M. J. Control of the mitogenicity of muramyl dipeptide. Int J Immunopharmacol. 1981;3(1):31–44. doi: 10.1016/0192-0561(81)90043-6. [DOI] [PubMed] [Google Scholar]
- Zighelboim J., Lichtenstein A., Benjamin D. Response of normal subjects to mitogens. I. Influence of adherent cells. Clin Immunol Immunopathol. 1981 Jun;19(3):406–415. doi: 10.1016/0090-1229(81)90083-0. [DOI] [PubMed] [Google Scholar]
- van Eden W., de Vries R. R., Stanford J. L., Rook G. A. HLA-DR3 associated genetic control of response to multiple skin tests with new tuberculins. Clin Exp Immunol. 1983 May;52(2):287–292. [PMC free article] [PubMed] [Google Scholar]



