Abstract
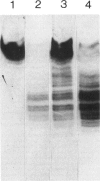
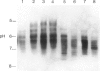
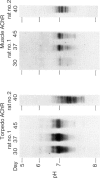
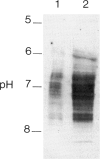
We have studied the isoelectric focusing pattern of antibodies expressed in rats with experimental autoimmune myasthenia gravis (EAMG) induced by immunization with acetylcholine receptors (AChR) purified from Torpedo californica. Sera or tissue eluates were obtained at intervals in the course of disease and subjected to isoelectric focusing. Subsequently, the focused antibodies were detected by autoradiography of gels labelled with 125I-alpha-bungarotoxin conjugated AChR. Reverse electrofocusing was used to separate complexes of antibody and AChR formed in vivo, thereby allowing detection of the full spectrotype (banding pattern). As little as 1.1 X 10(-12) moles of monoclonal antibodies (MoAbs) to AChR yielded distinct bands of radiolabelled antigen binding by this technique. The anti-AChR MoAbs studied showed a multitude of bands localized in neutral to alkaline position. The clonotypes expressed in late post-immunization sera were compared to early sera. The spectrotypes of immunized Lewis and Brown Norway rats were not identical. In early sera most of the isoelectric focusing bands were specific for T. californica AChR, whereas in late sera further expansion of the repertoire produced bands that reacted with rat muscle AChR as well. The focused bands that bound rat AChR also bound T. californica AChR. The anti-AChR antibodies eluted from muscles of rats with EAMG showed similar binding patterns to anti-receptor antibodies in rats' sera. These results indicate that the antibody specificities detected in serum are the same specificities which are effective in binding to muscle AChR in vivo. Minor specificities of serum anti-receptor antibodies are not disproportionally represented in the antibodies actually bound at the neuromuscular junction in EAMG.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almon R. R., Appel S. H. Serum acetylcholine-receptor antibodies in myasthenia gravis. Ann N Y Acad Sci. 1976;274:235–243. doi: 10.1111/j.1749-6632.1976.tb47689.x. [DOI] [PubMed] [Google Scholar]
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Patrick J. Linkage between the frequency of muscular weakness and loci that regulate immune responsiveness in murine experimental myasthenia gravis. J Exp Med. 1980 Sep 1;152(3):507–520. doi: 10.1084/jem.152.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Davie J. M. Detection of isoelectric focused antibody by autoradiography and hemolysis of antigen-coated erythrocytes. A comparison of methods. J Immunol Methods. 1975 Oct;8(4):363–372. doi: 10.1016/0022-1759(75)90058-7. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B., Tzartos S., Lindstrom J. Monoclonal antibodies as probes of acetylcholine receptor structure. 2. Binding to native receptor. Biochemistry. 1981 Apr 14;20(8):2181–2191. doi: 10.1021/bi00511a017. [DOI] [PubMed] [Google Scholar]
- Damle V. N., Karlin A. Affinity labeling of one of two alpha-neurotoxin binding sites in acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2039–2045. doi: 10.1021/bi00604a002. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Kao I. Effect of myasthenic patients' immunoglobulin on acetylcholine receptor turnover: selectivity of degradation process. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3422–3426. doi: 10.1073/pnas.75.7.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G., Lambert E. H., Howard F. M. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc. 1977 May;52(5):267–280. [PubMed] [Google Scholar]
- Engel A. G., Lindstrom J. M., Lambert E. H., Lennon V. A. Ultrastructural localization of the acetylcholine receptor in myasthenia gravis and in its experimental autoimmune model. Neurology. 1977 Apr;27(4):307–315. doi: 10.1212/wnl.27.4.307. [DOI] [PubMed] [Google Scholar]
- Fischbach M., Rabbie J., Talal N. Comparison of different antinucleic acid antibody spectrotypes in spontaneous, induced, and murine lupus. J Clin Invest. 1981 Oct;68(4):1036–1043. doi: 10.1172/JCI110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S., Bevan S., Kullberg R., Lindstrom J., Rice J. Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3090–3094. doi: 10.1073/pnas.74.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Myasthenic immunoglobulin accelerates acetylcholine receptor degradation. Science. 1977 Apr 29;196(4289):527–529. doi: 10.1126/science.850793. [DOI] [PubMed] [Google Scholar]
- Lefvert A. K., Bergström K. Acetylcholine receptor antibody in myasthenia gravis: purification and characterization. Scand J Immunol. 1978;8(6):525–533. doi: 10.1111/j.1365-3083.1978.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Einarson B. L., Lennon V. A., Seybold M. E. Pathological mechanisms in experimental autoimmune myasthenia gravis. I. Immunogenicity of syngeneic muscle acetylcholine receptor and quantitative extraction of receptor and antibody-receptor complexes from muscles of rats with experimental automimmune myasthenia gravis. J Exp Med. 1976 Sep 1;144(3):726–738. doi: 10.1084/jem.144.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Autoimmune response to acetylcholine receptors in myasthenia gravis and its animal model. Adv Immunol. 1979;27:1–50. doi: 10.1016/s0065-2776(08)60261-8. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Campbell M., Nave B. Specificities of antibodies to acetylcholine receptors. Muscle Nerve. 1978 Mar-Apr;1(2):140–145. doi: 10.1002/mus.880010206. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Merlie J. Immunization of rats with polypeptide chains from torpedo acetylcholine receptor causes an autoimmune response to receptors in rat muscle. Proc Natl Acad Sci U S A. 1978 Feb;75(2):769–773. doi: 10.1073/pnas.75.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Tzartos S. Production and assay of antibodies to acetylcholine receptors. Methods Enzymol. 1981;74(Pt 100):432–460. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Immunological studies of acetylcholine receptors. J Supramol Struct. 1976;4(3):389–403. doi: 10.1002/jss.400040310. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Nye L., De Carvalho L. P., Roitt I. M. An investigation of the clonality of human autoimmune thyroglobulin antibodies and their light chains. Clin Exp Immunol. 1981 Oct;46(1):161–170. [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Reed K., Vandlen R., Bode J., Duguid J., Raftery M. A. Characterization of acetylcholine receptor-rich and acetylcholinesterase-rich membrane particles from Torpedo californica electroplax. Arch Biochem Biophys. 1975 Mar;167(1):138–144. doi: 10.1016/0003-9861(75)90449-x. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Sahashi K., Engel A. G., Linstrom J. M., Lambert E. H., Lennon V. A. Ultrastructural localization of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J Neuropathol Exp Neurol. 1978 Mar-Apr;37(2):212–223. doi: 10.1097/00005072-197803000-00008. [DOI] [PubMed] [Google Scholar]
- Seybold M. E., Baergen R. N., Nave B., Lindstrom J. M. Anti-acetylcholine-receptor antibody concentrations after thymectomy in patients with myasthenia gravis. Br Med J. 1978 Oct 14;2(6144):1051–1053. doi: 10.1136/bmj.2.6144.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]