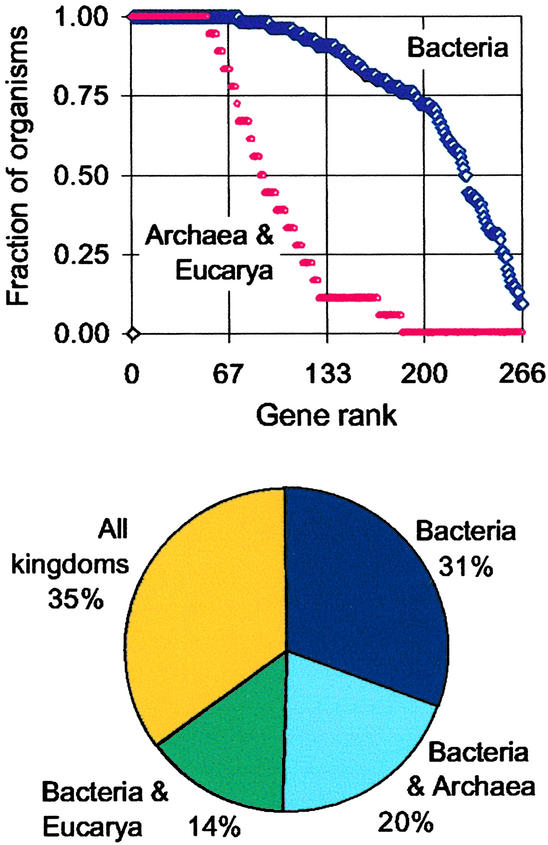
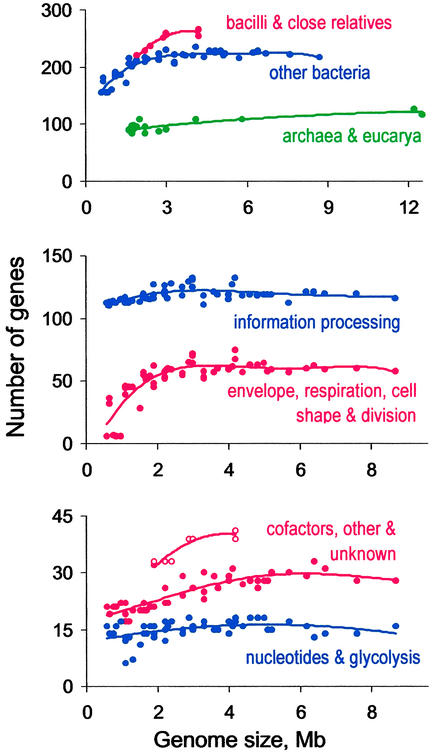
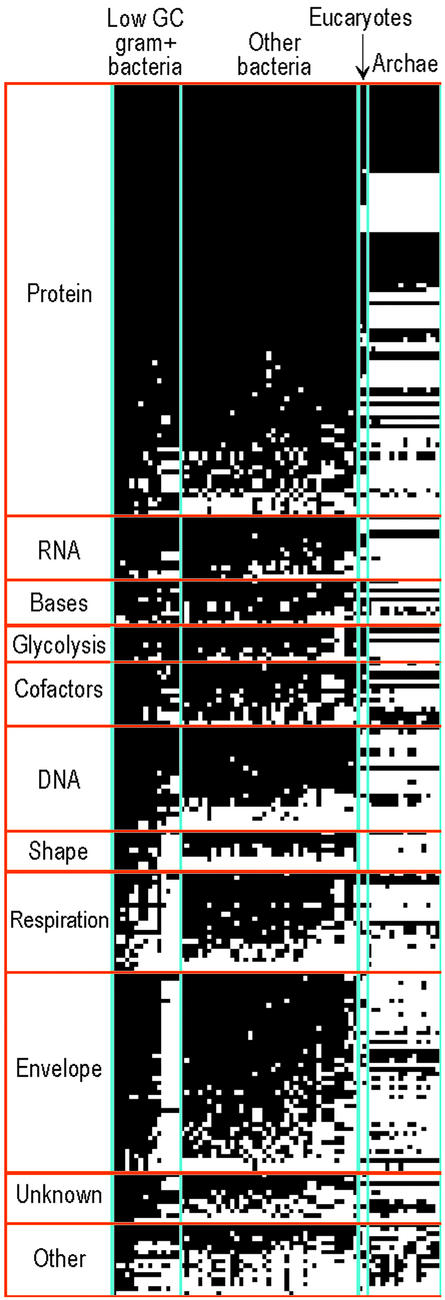
K Kobayashi
K Kobayashi
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,
S D Ehrlich
S D Ehrlich
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
b,c,
A Albertini
A Albertini
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
d,
G Amati
G Amati
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
d,
K K Andersen
K K Andersen
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
e,
M Arnaud
M Arnaud
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
f,
K Asai
K Asai
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
g,
S Ashikaga
S Ashikaga
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
h,
S Aymerich
S Aymerich
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
i,
P Bessieres
P Bessieres
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
j,
F Boland
F Boland
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
k,
S C Brignell
S C Brignell
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
l,
S Bron
S Bron
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
m,
K Bunai
K Bunai
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
n,
J Chapuis
J Chapuis
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
b,
L C Christiansen
L C Christiansen
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
o,
A Danchin
A Danchin
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
p,
M Débarbouillé
M Débarbouillé
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
f,
E Dervyn
E Dervyn
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
b,
E Deuerling
E Deuerling
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
q,
K Devine
K Devine
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
e,
S K Devine
S K Devine
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
e,
O Dreesen
O Dreesen
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
p,
J Errington
J Errington
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
r,
S Fillinger
S Fillinger
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
i,
S J Foster
S J Foster
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
k,
Y Fujita
Y Fujita
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
s,
A Galizzi
A Galizzi
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
d,
R Gardan
R Gardan
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
f,
C Eschevins
C Eschevins
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
m,
T Fukushima
T Fukushima
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
t,
K Haga
K Haga
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
u,
C R Harwood
C R Harwood
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
l,
M Hecker
M Hecker
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
v,
D Hosoya
D Hosoya
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
w,
M F Hullo
M F Hullo
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
p,
H Kakeshita
H Kakeshita
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
n,
D Karamata
D Karamata
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
x,
Y Kasahara
Y Kasahara
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,
F Kawamura
F Kawamura
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
h,
K Koga
K Koga
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
h,
P Koski
P Koski
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
y,
R Kuwana
R Kuwana
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
z,
D Imamura
D Imamura
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
w,
M Ishimaru
M Ishimaru
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
w,
S Ishikawa
S Ishikawa
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
t,
I Ishio
I Ishio
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
s,
D Le Coq
D Le Coq
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
i,
A Masson
A Masson
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,a,
C Mauël
C Mauël
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
x,
R Meima
R Meima
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
m,
R P Mellado
R P Mellado
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
b,b,
A Moir
A Moir
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
k,
S Moriya
S Moriya
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,
E Nagakawa
E Nagakawa
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
s,
H Nanamiya
H Nanamiya
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
h,
S Nakai
S Nakai
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,
P Nygaard
P Nygaard
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
o,
M Ogura
c,c,
T Ohanan
T Ohanan
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
q,
M O'Reilly
M O'Reilly
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
e,
M O'Rourke
M O'Rourke
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
k,
Z Pragai
Z Pragai
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
l,
H M Pooley
H M Pooley
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
x,
G Rapoport
G Rapoport
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
f,
J P Rawlins
J P Rawlins
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
r,
L A Rivas
L A Rivas
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
b,b,
C Rivolta
C Rivolta
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
x,
A Sadaie
A Sadaie
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
u,
Y Sadaie
Y Sadaie
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
g,
M Sarvas
M Sarvas
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
y,
T Sato
T Sato
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
w,
H H Saxild
H H Saxild
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
o,
E Scanlan
E Scanlan
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
e,
W Schumann
W Schumann
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
q,
J F M L Seegers
J F M L Seegers
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,a,
J Sekiguchi
J Sekiguchi
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
t,
A Sekowska
A Sekowska
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
p,
S J Séror
S J Séror
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a,a,
M Simon
M Simon
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
d,d,
P Stragier
P Stragier
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
d,d,
R Studer
R Studer
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
x,
H Takamatsu
H Takamatsu
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
z,
T Tanaka
c,c,
M Takeuchi
M Takeuchi
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
w,
H B Thomaides
H B Thomaides
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
r,
V Vagner
V Vagner
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
b,
J M van Dijl
J M van Dijl
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
m,
K Watabe
K Watabe
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
z,
A Wipat
A Wipat
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
l,
H Yamamoto
H Yamamoto
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
t,
M Yamamoto
M Yamamoto
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
s,
Y Yamamoto
Y Yamamoto
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
s,
K Yamane
K Yamane
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
n,
K Yata
K Yata
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
e,e,
K Yoshida
K Yoshida
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
s,
H Yoshikawa
H Yoshikawa
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
u,
U Zuber
U Zuber
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
v,
N Ogasawara
N Ogasawara
aGraduate School of Information Science, Nara Institute of Science and Technology, Nara 630-0101, Japan; bGénétique Microbienne, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; dGenetica e Microbiologia, Università di Pavia, 1 via Ferrata, 27100 Pavia, Italy; eGenetics, Smurfit Institute, Trinity College, Dublin 2, Ireland; fBiochimie Microbienne, Institut Pasteur, 25 Rue du Dr. Roux, 75015 Paris, France; gFaculty of Science, Saitama University, Saitama 338-8570, Japan; hCollege of Science, Rikkyo (St. Paul's) University, Tokyo 171-8501, Japan; iGénétique Moléculaire et Cellulaire, Institut National de la Recherche Agronomique–Centre National de la Recherche Scientifique–Institut National Agronomique Paris-Grignon, 78850 Thiverval-Grignon, France; jMathématiques Informatique Génomes, Institut National de la Recherche Agronomique, 78530 Jouy en Josas, France; kMolecular Biology and Biotechnology, University of Sheffield, Sheffield S10 2TN, United Kingdom; lCell and Molecular Bioscience, Newcastle University Medical School, Framlington Place, Newcastle upon Tyne NE2 4HH, United Kingdom; mGenetics, Groningen Biomolecular Sciences and Biotechnology Institute, 9750 AA, Haren, The Netherlands; nInstitute of Biological Sciences, University of Tsukuba, Ibaraki 305-8572, Japan; oBiological Chemistry, Institute of Molecular Biology, Solvgade 83, 1307 K, Copenhagen, Denmark; pGenetique des Genomes Bacteriens, Institut Pasteur, Unité de Recherche Associée, Centre National de la Recherche Scientifique 2171, 75015 Paris, France; qInstitute of Genetics, Bayreuth University, D-95440 Bayreuth, Germany; rSir William Dunn School of Pathology, Oxford University, Oxford OX1 3RE, United Kingdom; sFaculty of Life Science and Biotechnology, Fukuyama University, Hiroshima 729-0292, Japan; tFaculty of Textile Science and Technology, Shinshu University, Nagano 386-8564, Japan; uDepartment of Bioscience, Tokyo University of Agriculture, Tokyo 156-8502, Japan; vInstitute for Microbiology, Ernst-Moritz-Arndt-University, D-17487 Greifswald, Germany; wDepartment of International Environmental and Agricultural Science, Tokyo University of Agriculture and Technology, Tokyo 183-8509, Japan; xInstitut de Genetique et de Biologie Microbiennes, CH-1005 Lausanne, Switzerland; yNational Public Health Institute, 00300, Helsinki, Finland; zFaculty of Pharmaceutical Sciences, Setsunan University, Osaka 573-0101, Japan; aaInstitut de Génétique et Microbiologie, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8621, Université Paris-Sud, 91405 Orsay Cedex, France; bbCentro Nacional de Biotecnología, Campus de la Universidad Autónoma, Cantoblanco, 28049 Madrid, Spain; ccSchool of Marine Science and Technology, University of Tokai, Shizuoka 424-8610, Japan; ddInstitut de Biologie Physico-Chimique, 75005 Paris, France; and eeRadioisotope Center, National Institute of Genetics, Shizuoka 411-8540, Japan
a