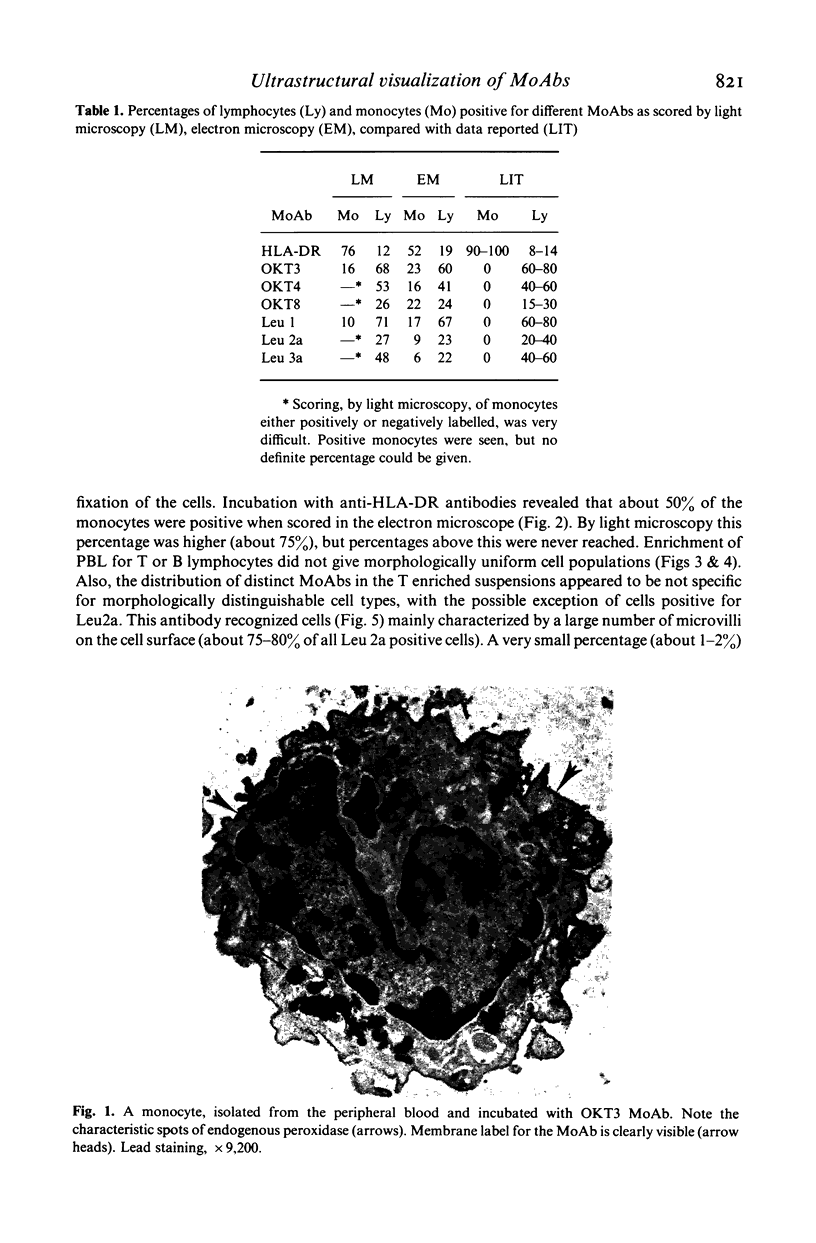
Abstract
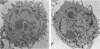
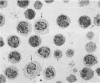
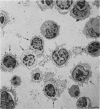
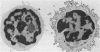
Human peripheral blood mononuclear cell (PBMC) were treated with a panel of monoclonal antibodies (MoAbs) for the demonstration of membrane antigens at the ultrastructural level. The bound MoAbs were linked by rabbit anti-mouse IgG to a peroxidase-anti-peroxidase (PAP) complex composed of monoclonal mouse anti-peroxidase antibodies and horse radish peroxidase. This labelling method with a three step incubation procedure resulted in clear demonstration of the membrane antigens. Moreover, the use of the PAP complex as marker permitted the recognition of monocytes not only by morphology but also by their endogenous peroxidase pattern. In addition, it was observed that the MoAbs used, supposedly specific for T lymphocytes, reacted to a certain degree with monocytes.
Full text
PDF

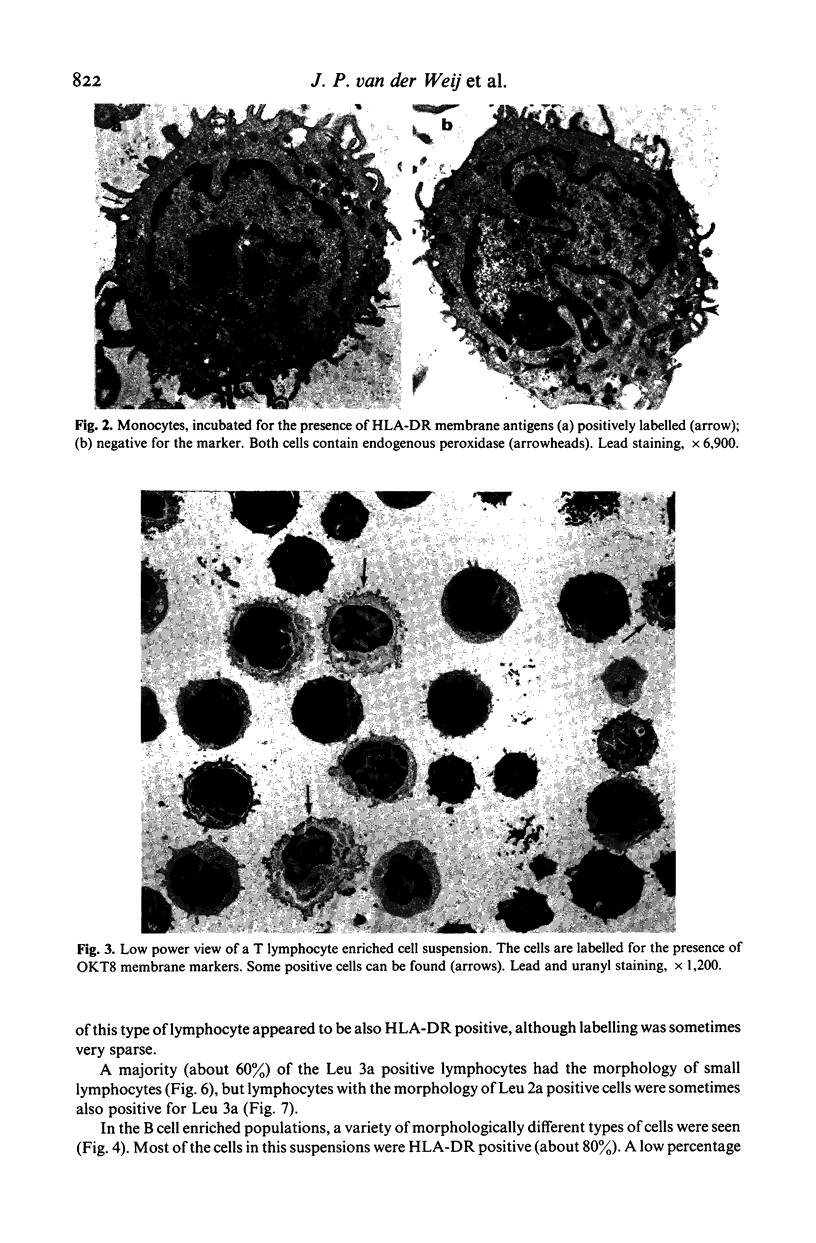
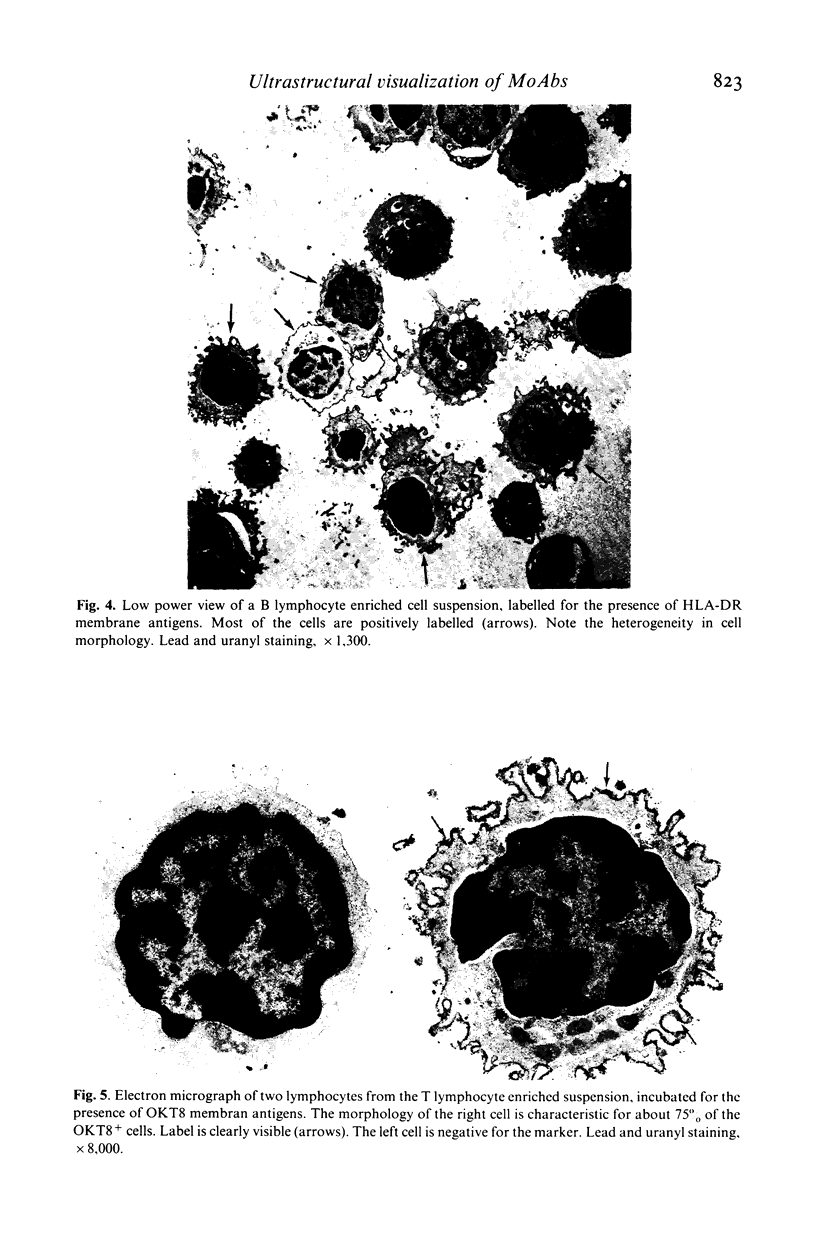
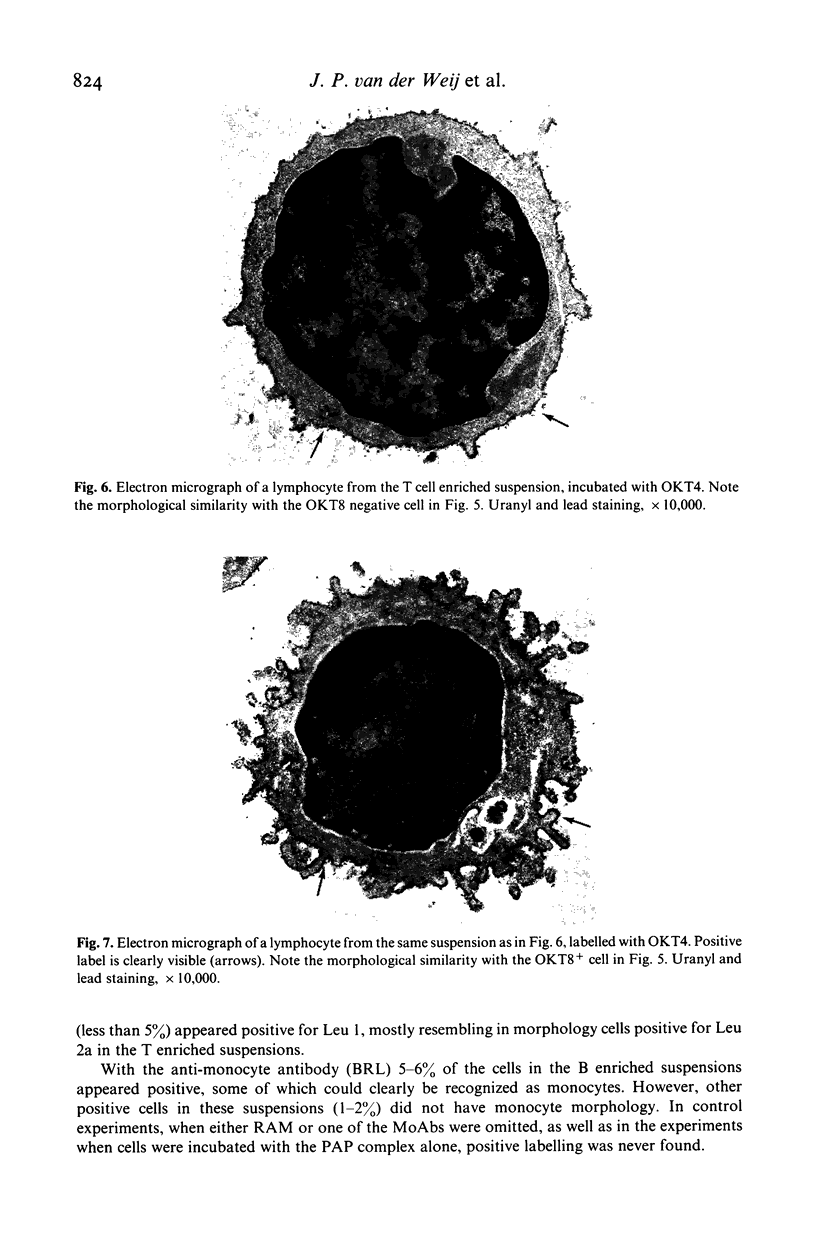


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cnossen J., Lafeber G. J., Damsteeg W. J., Meijer C. J. Mixed rosette assay for the detection of T mu and T gamma lymphocytes. J Immunol Methods. 1980;36(3-4):197–209. doi: 10.1016/0022-1759(80)90125-8. [DOI] [PubMed] [Google Scholar]
- Daems W. T., Brederoo P. Electron microscopical studies on the structure, phagocytic properties, and peroxidatic activity of resident and exudate peritoneal macrophages in the guinea pig. Z Zellforsch Mikrosk Anat. 1973 Nov 5;144(2):247–297. doi: 10.1007/BF00307305. [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Cadoni A., Franzi A. T., Ghigliotti C., Leprini A., Zicca A., Grossi C. E. Ultrastructure and cytochemistry of human peripheral blood lymphocytes. Similarities between the cells of the third population and TG lymphocytes. Eur J Immunol. 1980 Jul;10(7):562–570. doi: 10.1002/eji.1830100714. [DOI] [PubMed] [Google Scholar]
- Grossi C. E., Webb S. R., Zicca A., Lydyard P. M., Moretta L., Mingari M. C., Cooper M. D. Morphological and histochemical analyses of two human T-cell subpopulations bearing receptors for IgM or IgG. J Exp Med. 1978 May 1;147(5):1405–1417. doi: 10.1084/jem.147.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydyard P. M., Fanger M. W. Receptors for IgM on human lymphocytes. II. Mitogen-induced modulation of receptor expression. Clin Exp Immunol. 1979 Sep;37(3):486–494. [PMC free article] [PubMed] [Google Scholar]
- Matutes E., Catovsky D. The fine structure of normal lymphocyte subpopulations--a study with monoclonal antibodies and the immunogold technique. Clin Exp Immunol. 1982 Nov;50(2):416–425. [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. F., Bhan A. K., Sato S., Harrist T. J., Mihm M. C., Jr Characterization of Langerhans cells by the use of monoclonal antibodies. Lab Invest. 1981 Nov;45(5):465–468. [PubMed] [Google Scholar]
- Poore T. E., Barrett S. G., Kadin M. E., Bainton D. F. Ultrastructural localization of acid phosphatase in rosetted T and B lymphocytes of normal human blood. Am J Pathol. 1981 Jan;102(1):72–83. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Penta A. C., Hussey R. E., Schlossman S. F. A rapid method for separating functionally intact human T lymphocytes with monoclonal antibodies. Clin Immunol Immunopathol. 1981 Nov;21(2):257–266. doi: 10.1016/0090-1229(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W., Coffman R. C., Weissman I. L. Immunoelectron microscopy of cell surface antigens: a quantitative analysis of antibody binding after different fixation protocols. Histochem J. 1980 May;12(3):349–361. doi: 10.1007/BF01006955. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- de Vries E., van de Putte L. B., Lafeber G. J., van Buysen-Nutters A. C., de Vetten-van der Hulst H. L., Meyer C. J. Morphological differences in the interactions between human mononuclear cells and coated or uncoated sheep red blood cells: a freeze-etch study of different types of rosettes. Cell Tissue Res. 1976 Apr 28;168(1):79–88. doi: 10.1007/BF00219725. [DOI] [PubMed] [Google Scholar]
- van der Loo E. M., Cnossen J., Meijer C. J. Morphological aspects of T cell subpopulations in human blood: characterization of the cerebriform mononuclear cells in healthy individuals. Clin Exp Immunol. 1981 Mar;43(3):506–516. [PMC free article] [PubMed] [Google Scholar]