Abstract
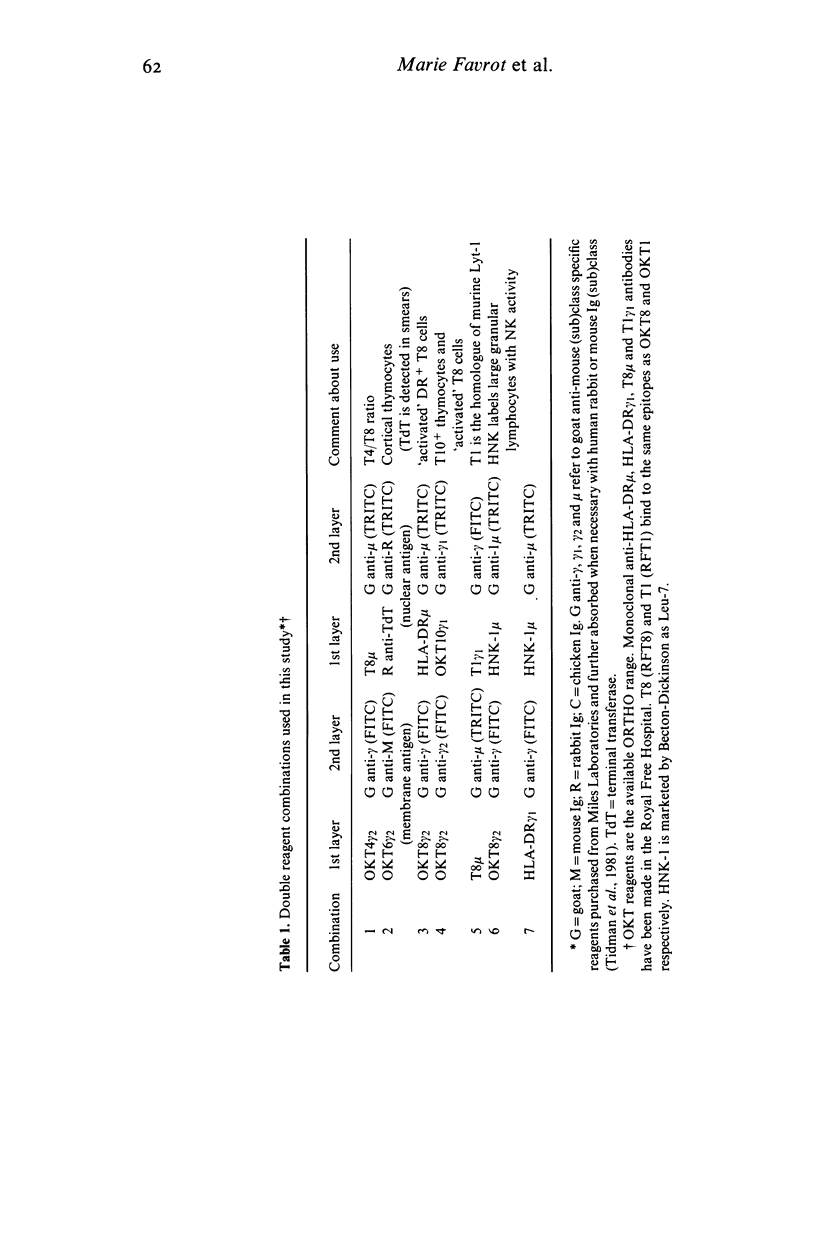
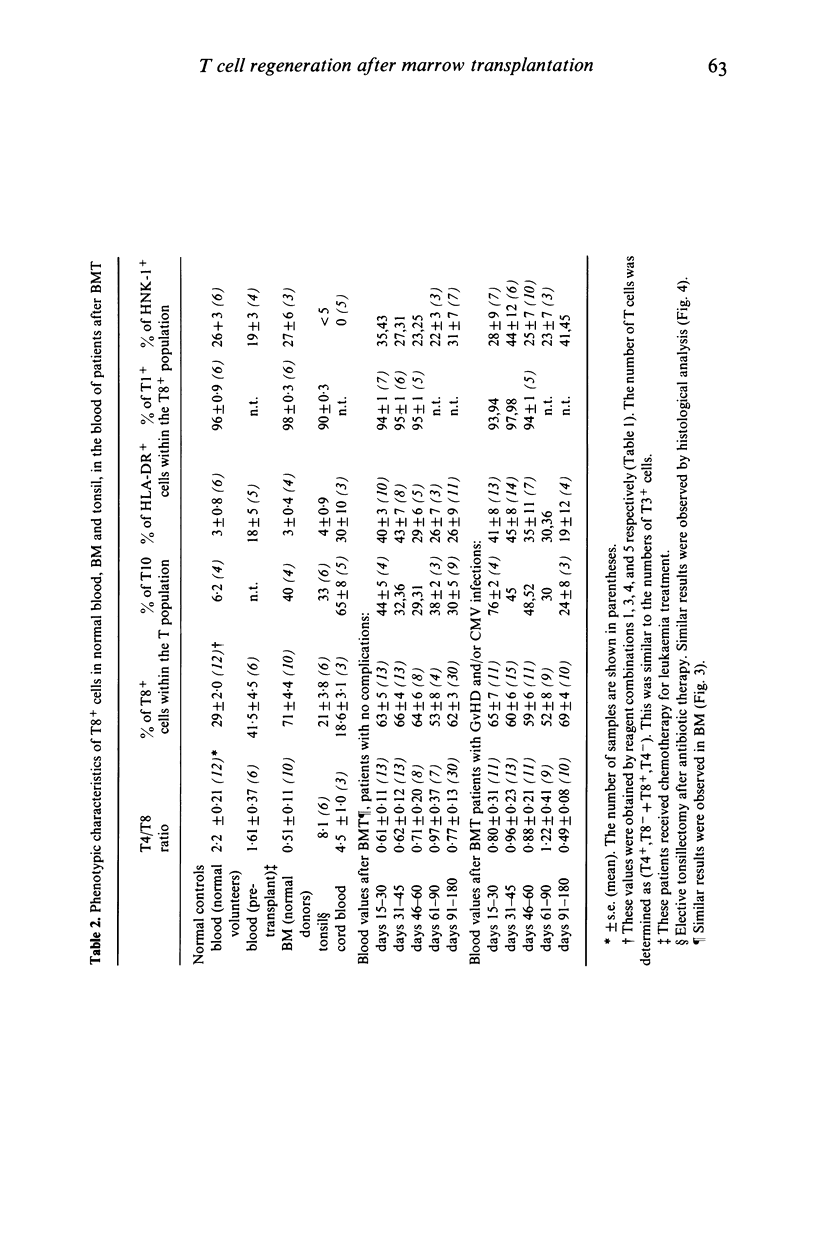
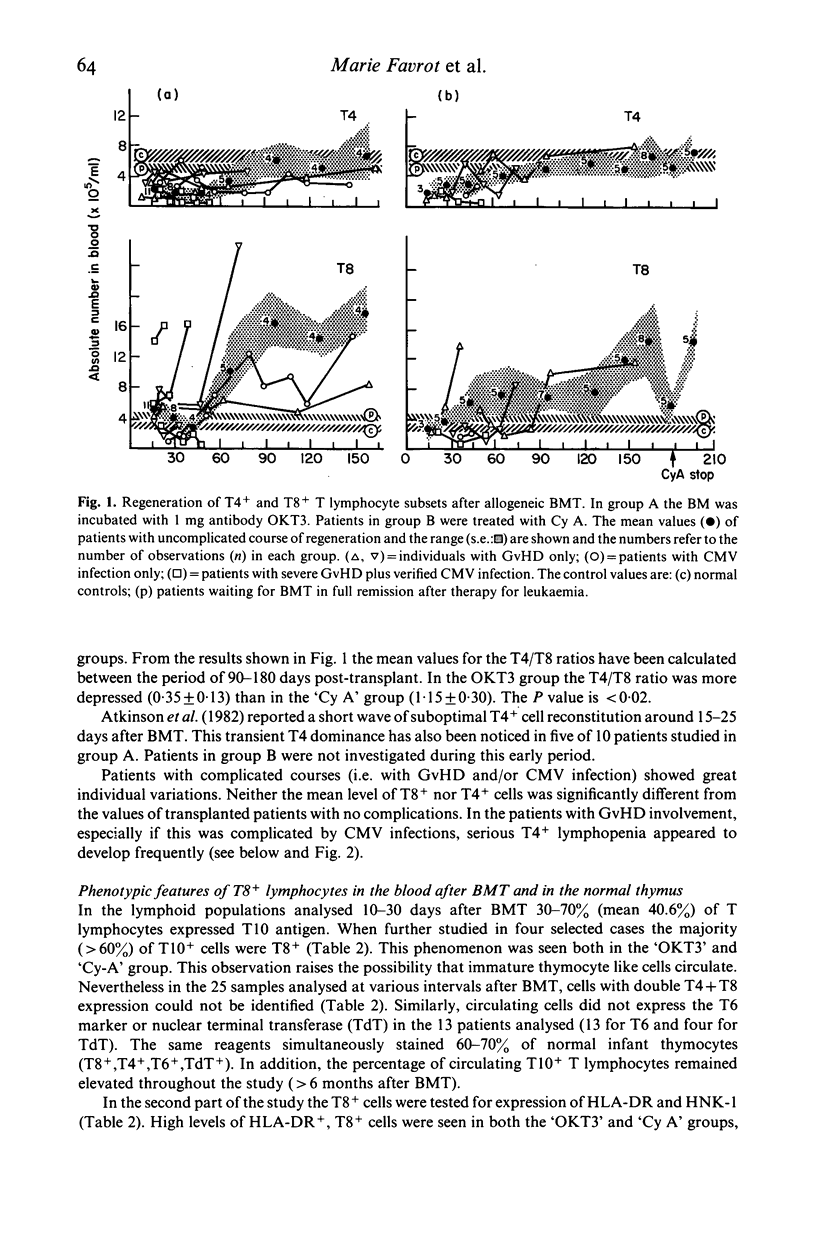
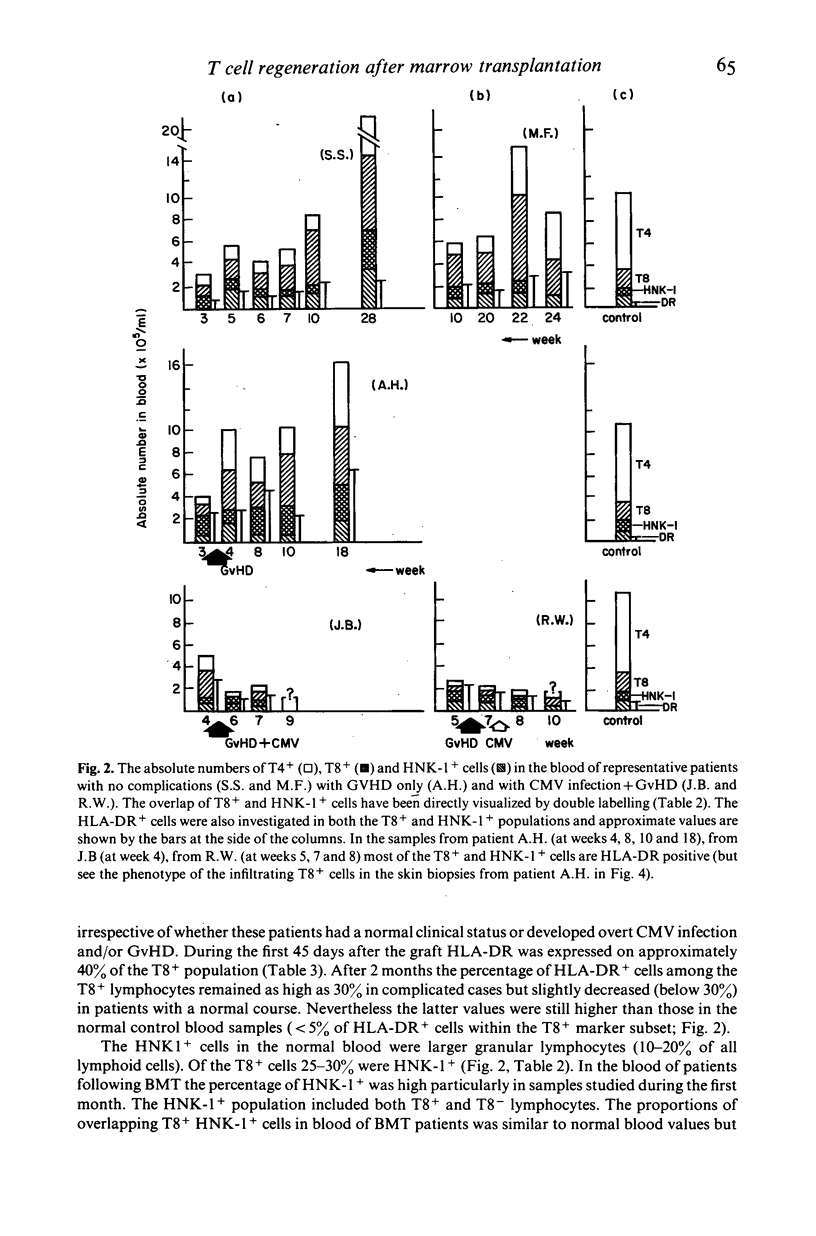
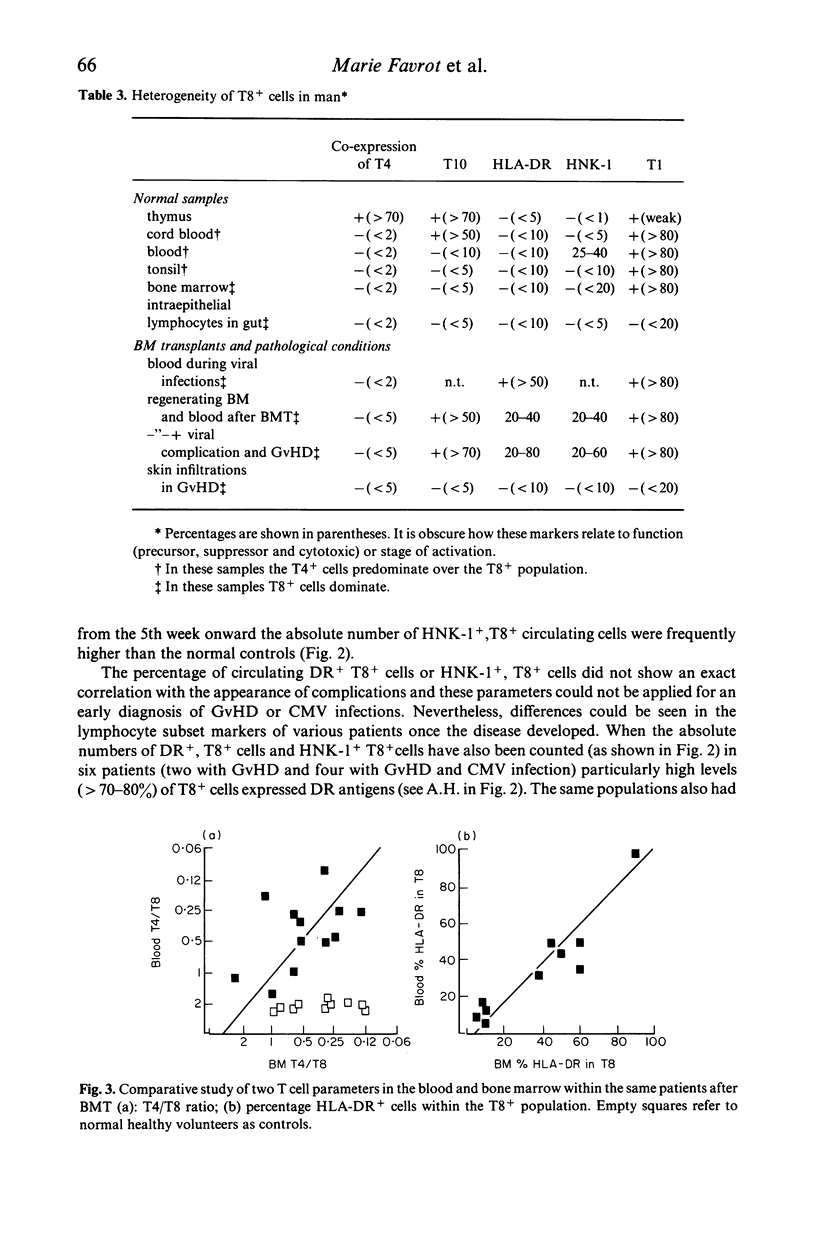
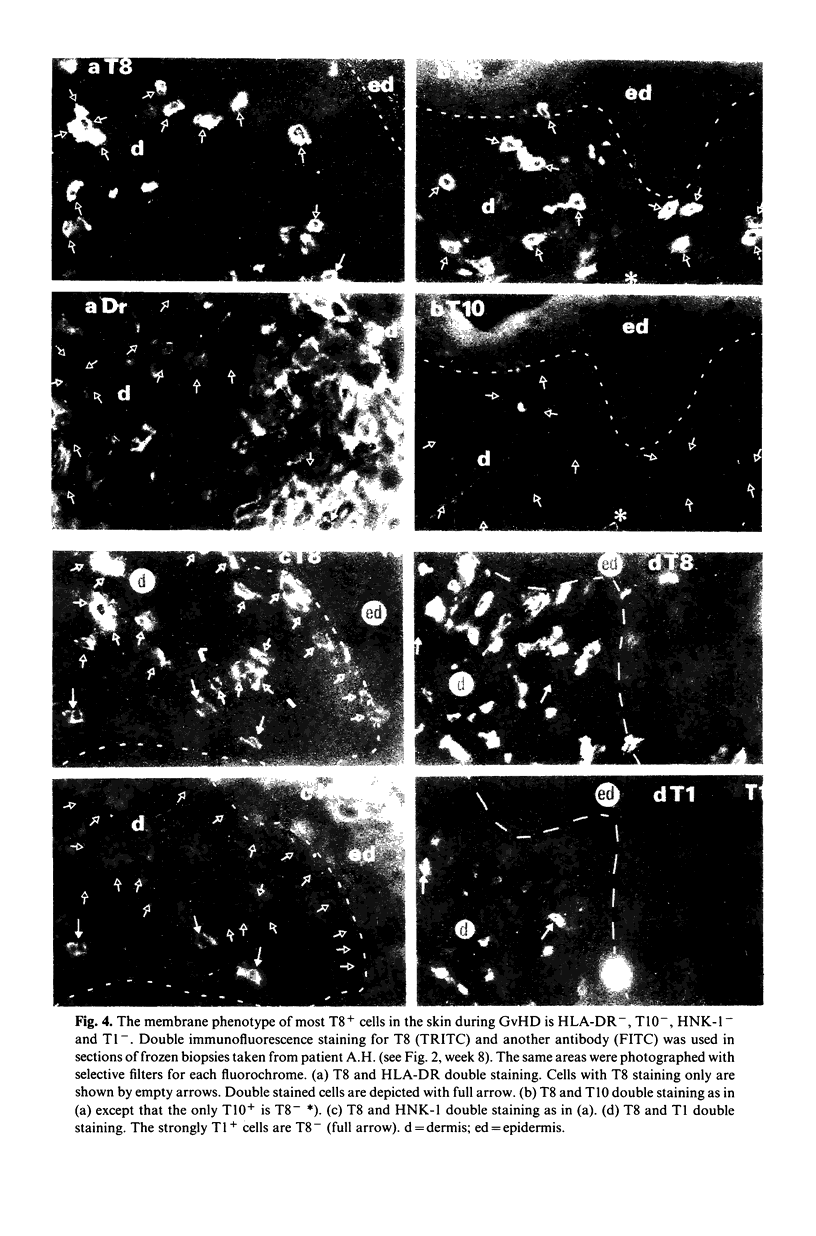
Various T cell subsets were characterized by double immunofluorescent staining using monoclonal antibodies (MoAb) in blood, bone marrow (BM) and tissues of 29 patients after allogeneic BM transplantation (BMT). In an attempt to prevent graft versus host disease (GvHD), 15 patients received cyclosporin A (Cy A). In the remaining 14 patients the BM was pre-incubated with a MoAb, OKT3. The regeneration of T4+ subset was delayed and the level of T8+ cells was abnormally high even 1 year after engraftment. This did not have any predictive value for the appearance of complications such as GvHD or severe viral infections. The number of T8+ cells was lower in the group of patients who received Cy A than in the OKT3 group (0·7±0·2 vs 1·5±0·3×109/1 at day 90). In contrast to normal individuals, the T4/T8 ratio in both blood and regenerating BM of BMT patients was <1. A sizeable subset of circulating T cells expressed the phenotype T8+,T10+,HNK-1+,DR+. Circulating cells of this phenotype were transiently very high (up to 50%) in patients with active GvHD or suffering from severe viral infection. This subpopulation of lymphocytes was not found in the epidermal infiltrate that accompanied GvHD where the predominant phenotype was T8+,T1-,T10-,HNK-1-,DR-. We conclude therefore that after BMT the number and phenotype of circulating T cells reflects the T cell distribution seen in the regenerating BM.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Atkinson K., Hansen J. A., Storb R., Goehle S., Goldstein G., Thomas E. D. T-cell subpopulations identified by monoclonal antibodies after human marrow transplantation. I. Helper-inducer and cytotoxic-suppressor subsets. Blood. 1982 Jun;59(6):1292–1298. [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Crawford D. H., Brickell P., Tidman N., McConnell I., Hoffbrand A. V., Janossy G. Increased numbers of cells with suppressor T cell phenotype in the peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol. 1981 Feb;43(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Friedrich W., O'Reilly R. J., Koziner B., Gebhard D. F., Jr, Good R. A., Evans R. L. T-lymphocyte reconstitution in recipients of bone marrow transplants with and without GVHD: imbalances of T-cell subpopulations having unique regulatory and cognitive functions. Blood. 1982 Apr;59(4):696–701. [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Kung P. C., Talle M. A., DeMaria M. E., Butler M. S., Lifter J., Goldstein G. Strategies for generating monoclonal antibodies defining human t-lymphocyte differentiation antigens. Transplant Proc. 1980 Sep;12(3 Suppl 1):141–146. [PubMed] [Google Scholar]
- Lampert I. A., Janossy G., Suitters A. J., Bofill M., Palmer S., Gordon-Smith E., Prentice H. G., Thomas J. A. Immunological analysis of the skin in graft versus host disease. Clin Exp Immunol. 1982 Oct;50(1):123–131. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G. Thymus-dependent and thymus-independent subpopulations of intestinal intraepithelial lymphocytes: a granular subpopulation of probable bone marrow origin and relationship to mucosal mast cells. Blood. 1980 Mar;55(3):532–535. [PubMed] [Google Scholar]
- Powles R. L., Clink H. M., Spence D., Morgenstern G., Watson J. G., Selby P. J., Woods M., Barrett A., Jameson B., Sloane J. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980 Feb 16;1(8164):327–329. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- Prentice H. G., Blacklock H. A., Janossy G., Bradstock K. F., Skeggs D., Goldstein G., Hoffbrand A. V. Use of anti-T-cell monoclonal antibody OKT3 to prevent acute graft-versus-host disease in allogeneic bone-marrow transplantation for acute leukaemia. Lancet. 1982 Mar 27;1(8274):700–703. doi: 10.1016/s0140-6736(82)92619-8. [DOI] [PubMed] [Google Scholar]
- Ramsay N. K., Kersey J. H., Robison L. L., McGlave P. B., Woods W. G., Krivit W., Kim T. H., Goldman A. I., Nesbit M. E., Jr A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med. 1982 Feb 18;306(7):392–397. doi: 10.1056/NEJM198202183060703. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Saral R., Burns W. H., Laskin O. L., Santos G. W., Lietman P. S. Acyclovir prophylaxis of herpes-simplex-virus infections. N Engl J Med. 1981 Jul 9;305(2):63–67. doi: 10.1056/NEJM198107093050202. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Goldstein G., Jewell D. P. T lymphocyte subsets in human intestinal mucosa: the distribution and relationship to MHC-derived antigens. Clin Exp Immunol. 1981 Jun;44(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Thomas E. D., Storb R., Clift R. A., Fefer A., Johnson L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (second of two parts). N Engl J Med. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- Tidman N., Janossy G., Bodger M., Granger S., Kung P. C., Goldstein G. Delineation of human thymocyte differentiation pathways utilizing double-staining techniques with monoclonal antibodies. Clin Exp Immunol. 1981 Sep;45(3):457–467. [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Kronke M., Solbach W., Scheurich P., Röllinghoff M., Pfizenmaier K. Murine T cell subsets and interleukins: relationships between cytotoxic T cells, helper T cells and accessory cells. Clin Haematol. 1982 Oct;11(3):607–630. [PubMed] [Google Scholar]
- de Bruin H. G., Astaldi A., Leupers T., van de Griend R. J., Dooren L. J., Schellekens P. T., Tanke H. J., Roos M., Vossen J. M. T lymphocyte characteristics in bone marrow-transplanted patients. II. Analysis with monoclonal antibodies. J Immunol. 1981 Jul;127(1):244–251. [PubMed] [Google Scholar]
- van de Griend R. J., Astaldi A., Vossen J. M., Dooren L. J., Schellekens P. T., Zwaan F. E., van den Ende A., Roos M., Roos D. T lymphocyte characteristics in bone marrow-transplanted patients. I. Changes in biochemical properties that correlate with the immunologic reconstitution. J Immunol. 1981 Feb;126(2):636–640. [PubMed] [Google Scholar]


