Abstract
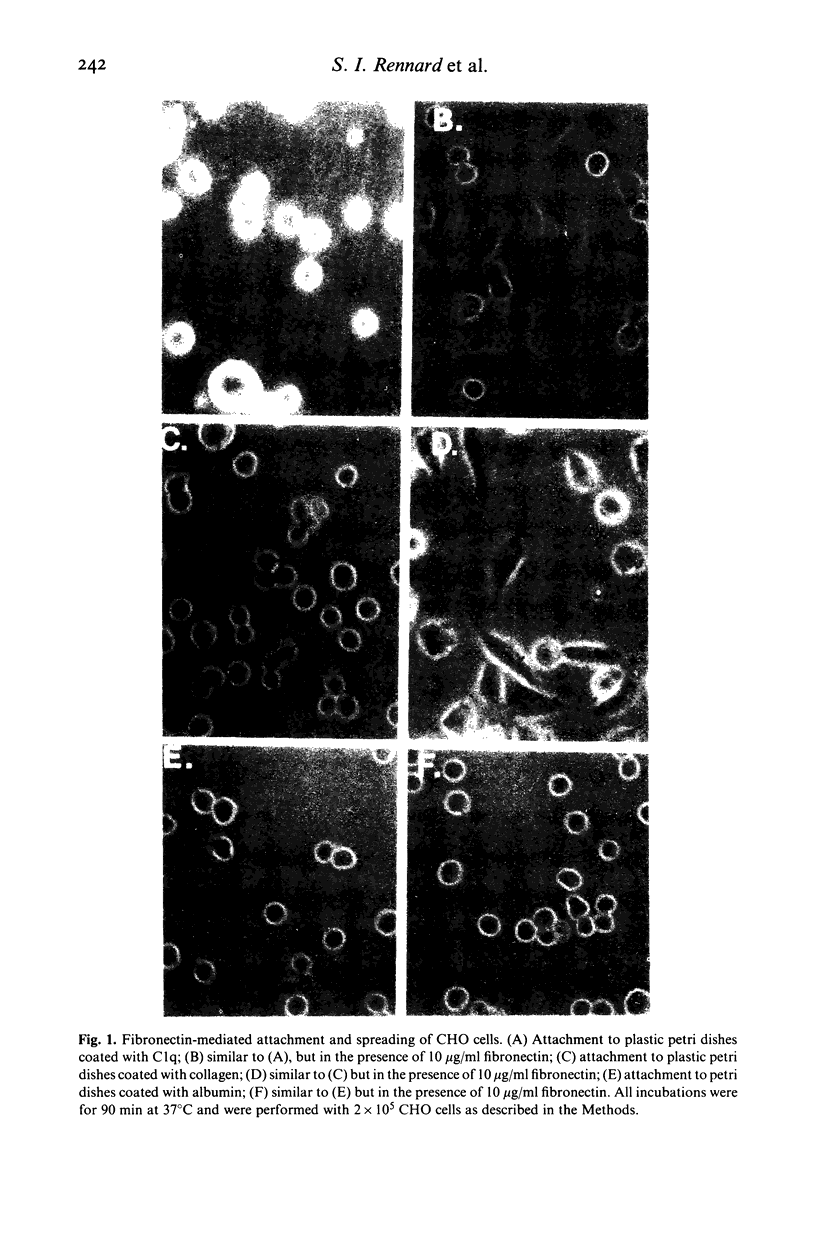
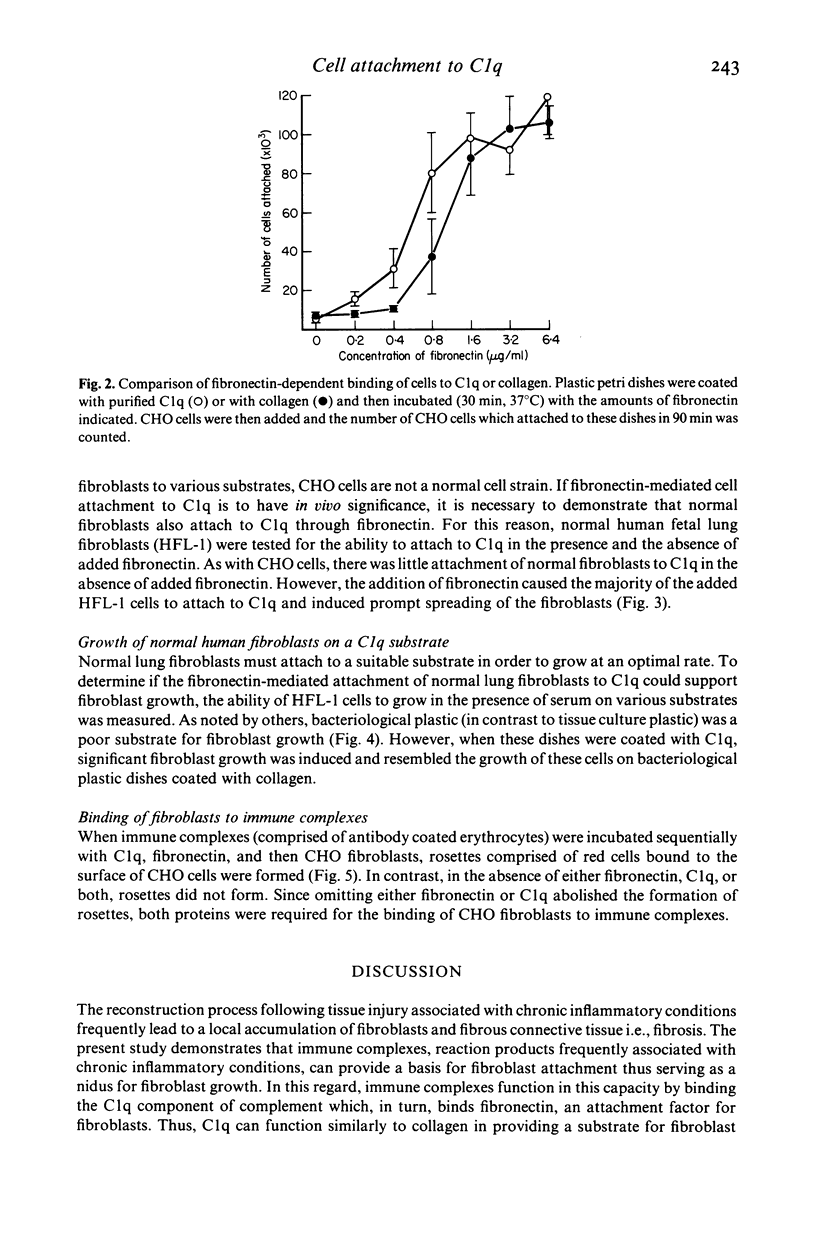
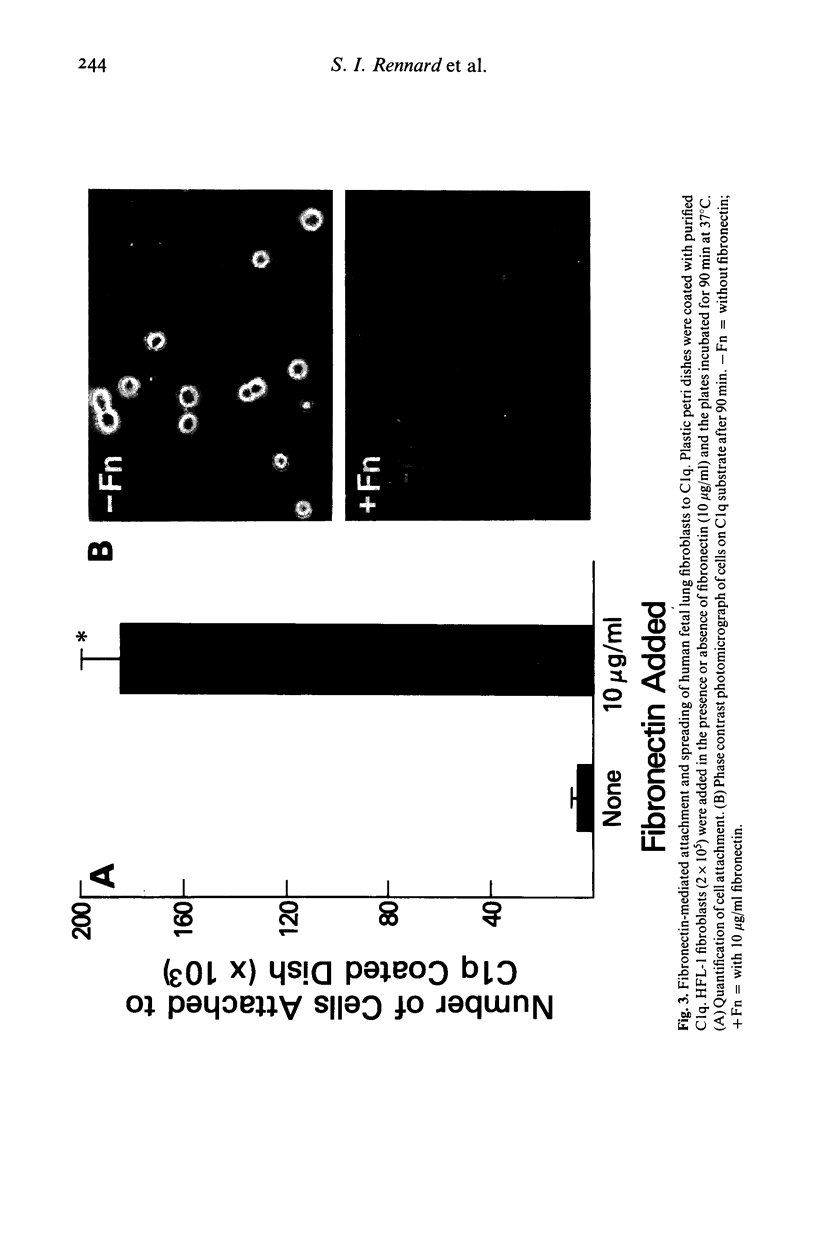
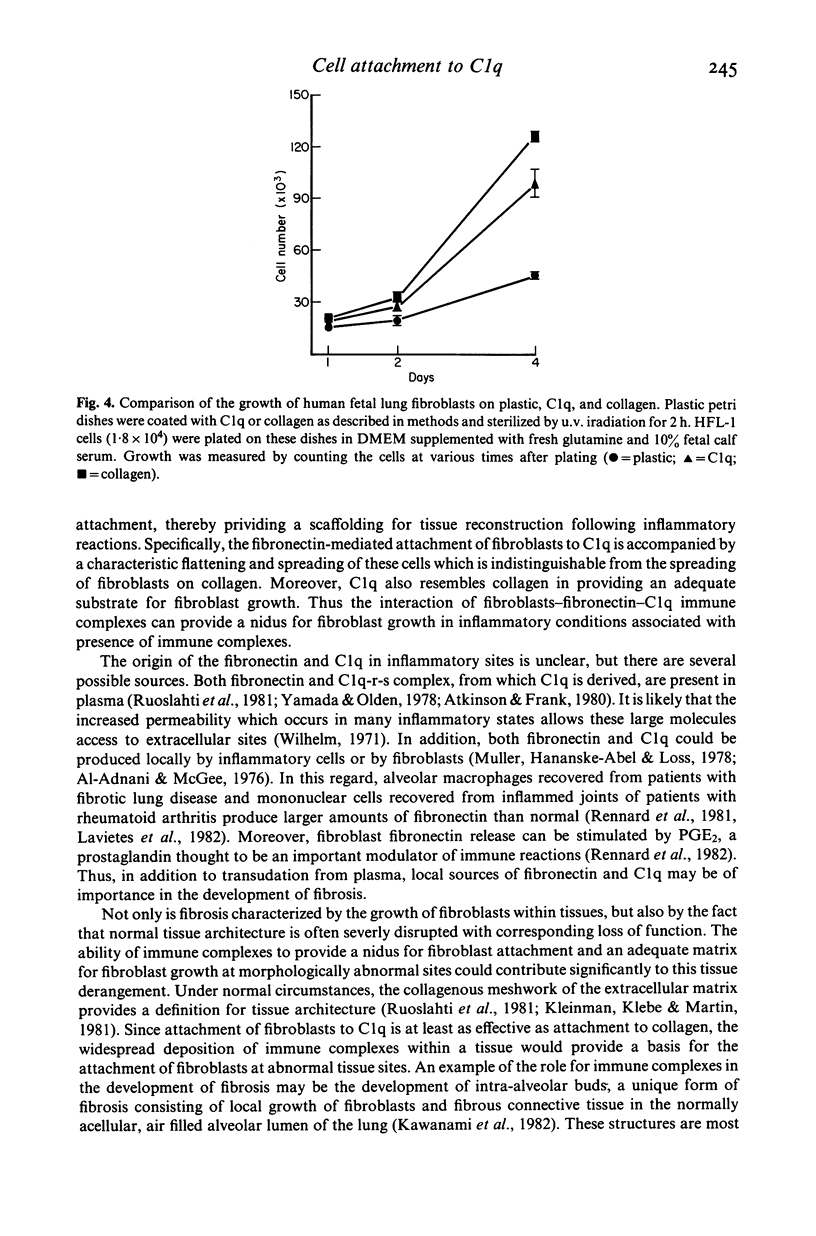
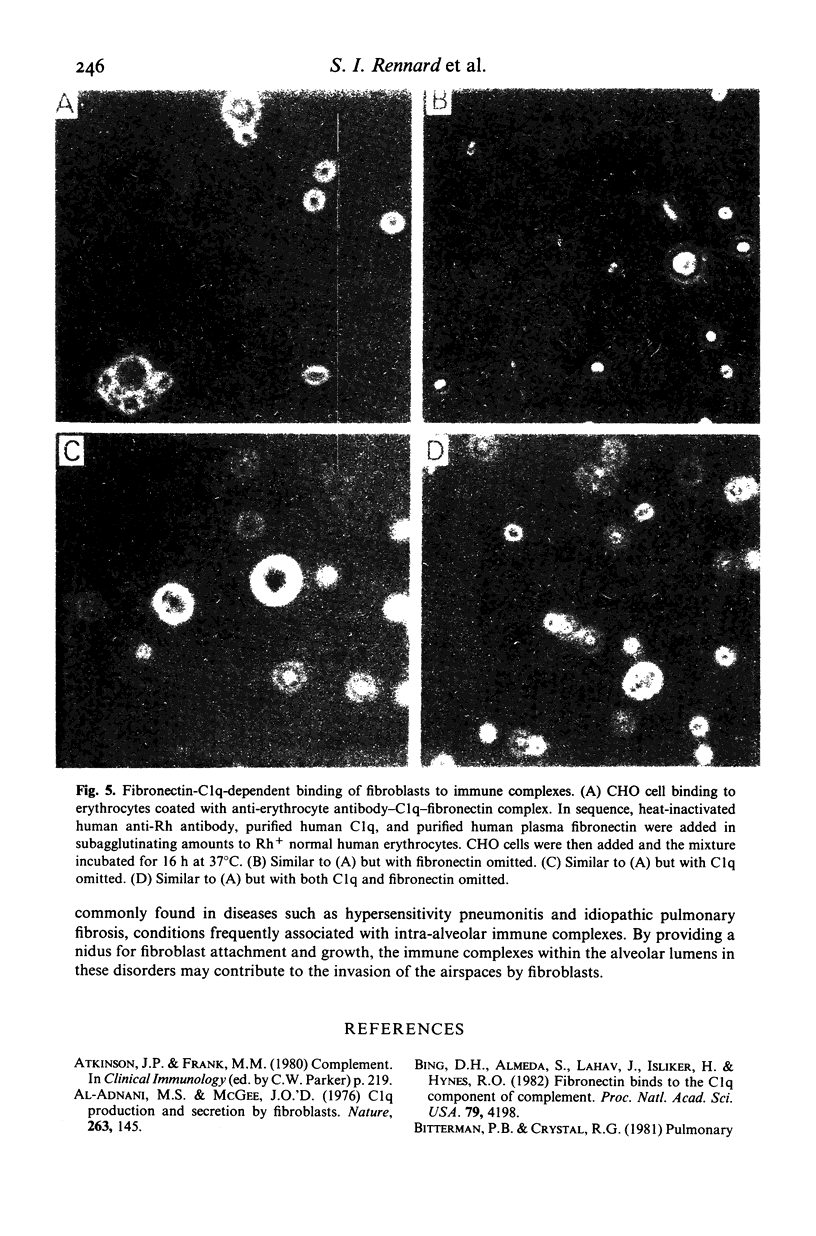
Chronic inflammatory processes frequently lead to the abnormal replacement of normal tissue elements by increased numbers of fibroblasts and fibrous connective tissue, i.e., fibrosis. Since the growth of fibroblasts requires that these cells be attached to an extracellular support, the current study was designed to determine if the interaction between the fibroblast attachment factor fibronectin and the C1q component of complement could support fibroblast attachment and growth and thus could form a basis for the attachment of fibroblasts in abnormal tissue locations in those inflammatory states where C1q is bound. Fibronectin purified from human plasma supported attachment of both Chinese hamster ovary cells and of normal fetal lung fibroblasts (HFL-1) to C1q coated substrates. The attachment activity was approximately twice that of attachment to collagen, and was specific, as no attachment occurred to albumin coated substrates. Cells attached to C1q substrates demonstrated characteristic 'spreading' similar to those on collagen. Moreover, the C1q substrate resembled collagen in its ability to support fibroblast growth. Further, the ability of the interaction between C1q and fibronectin to mediate attachment of fibroblasts to immune complexes was demonstrated by the formation of fibroblast-red blood cell-immune complex rosettes, a process that was dependent on both fibronectin and C1q. Thus, the interaction between fibronectin and C1q could serve as the basis for fibroblast attachment and growth in abnormal tissue sites where immune complexes are formed and could be a contributing factor to the development of fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Adnani M. S., McGee J. O. Clq production and secretion by fibroblasts. Nature. 1976 Sep 9;263(5573):145–146. doi: 10.1038/263145a0. [DOI] [PubMed] [Google Scholar]
- Bing D. H., Almeda S., Isliker H., Lahav J., Hynes R. O. Fibronectin binds to the C1q component of complement. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4198–4201. doi: 10.1073/pnas.79.13.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Menzel E. J., Smolen J. S., Liotta L., Reid K. B. Interaction of fibronectin with C1q and its collagen-like fragment (CLF). FEBS Lett. 1981 Jun 29;129(1):188–192. doi: 10.1016/0014-5793(81)80787-9. [DOI] [PubMed] [Google Scholar]
- Müller W., Hanauske-Abel H., Loos M. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J Immunol. 1978 Oct;121(4):1578–1584. [PubMed] [Google Scholar]
- Onal E., Lopata M., O'Connor T. Pathogenesis of apneas in hypersomnia-sleep apnea syndrome. Am Rev Respir Dis. 1982 Feb;125(2):167–174. doi: 10.1164/arrd.1982.125.2.167. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Sorvillo J., Gigli I. The interaction of human plasma fibronectin with a subunit of the first component of complement, C1q. J Immunol. 1982 May;128(5):2036–2039. [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. Generation of a fibroblast chemotactic factor in serum by activation of complement. J Clin Invest. 1979 Nov;64(5):1379–1385. doi: 10.1172/JCI109595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M. Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J Immunol. 1971 Feb;106(2):304–313. [PubMed] [Google Scholar]