Abstract
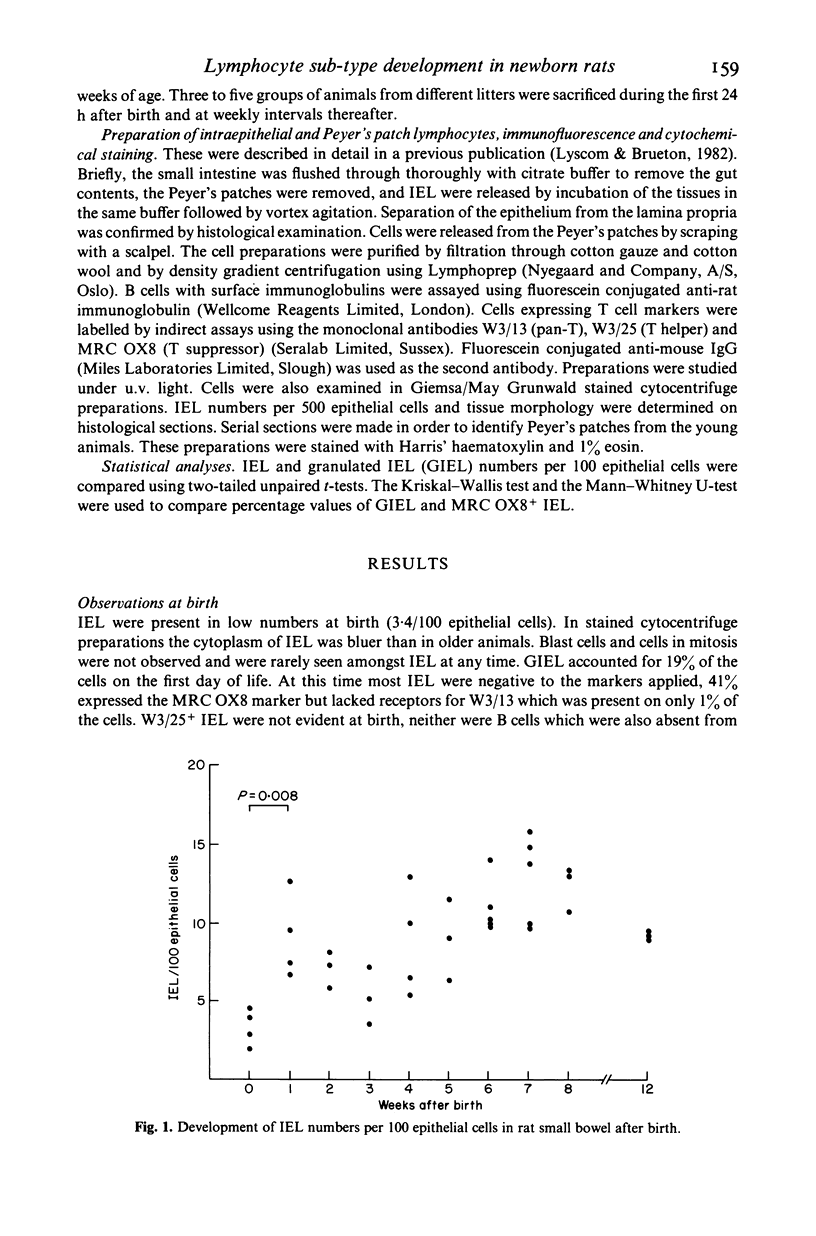
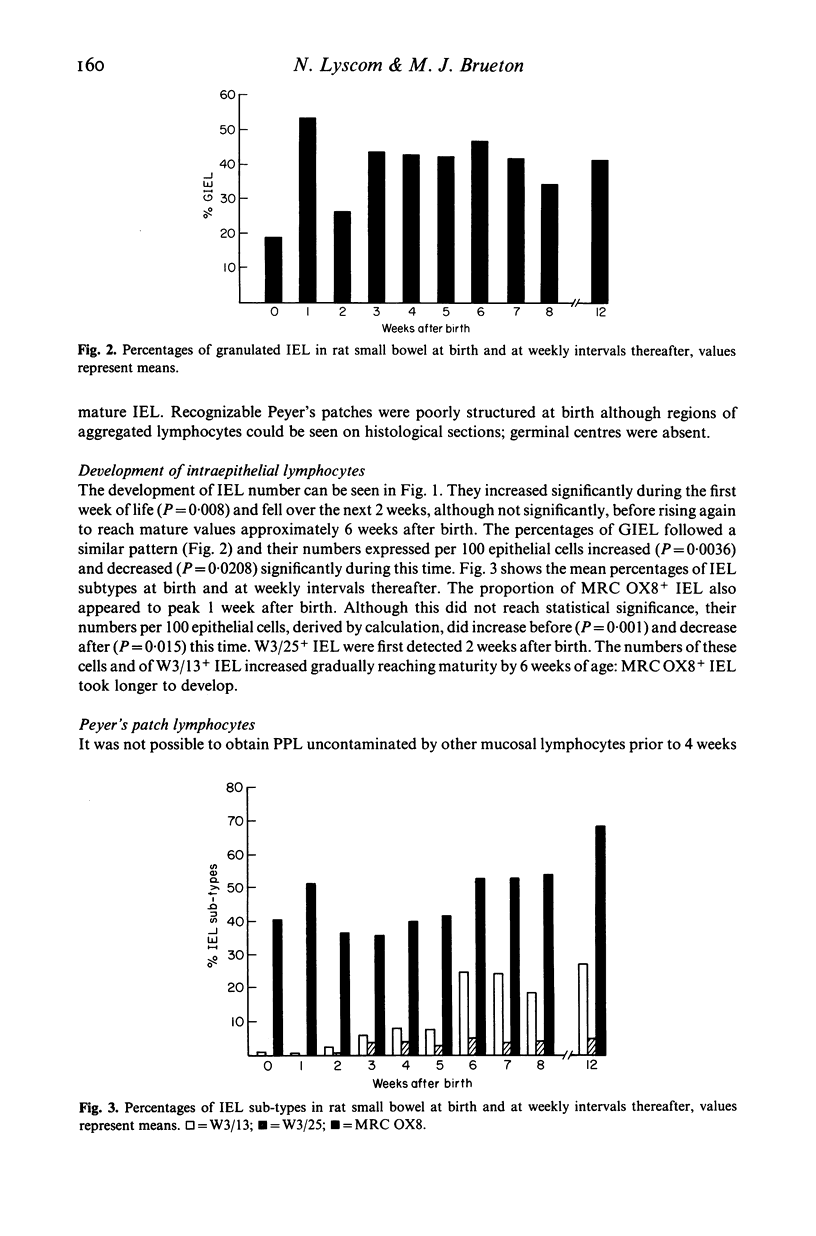
Small intestinal intraepithelial (IEL) and Peyer's patch (PPL) lymphocytes of newborn rats have been studied in histological sections and in isolated cell suspensions. Initially IEL numbers were low compared with older animals, and fewer cells were labelled by the monoclonal antibody markers used. At birth, 41% of IEL expressed receptors for MRC OX8 (T suppressor marker) yet lacked receptors for W3/13 (pan-T marker) which was present on only 1% of these cells. IEL with receptors for W3/25 (T helper marker) were not seen until 2 weeks of age. Granulated cells (GIEL) accounted for only 19% of IEL at birth. The numbers and relative proportions of these lymphocyte sub-types were still not mature at the time of weaning. In the first week of life there was a short lived upsurge in numbers of GIEL and MRC OX8+ IEL. The relative distribution of PPL sub-types was mature 4 weeks after birth, but the morphology of this tissue did not appear to be well differentiated until 1 week later.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud-Battandier F., Bundy B. M., O'Neill M., Bienenstock J., Nelson D. L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J Immunol. 1978 Sep;121(3):1059–1065. [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Flannery G. R. Quantitative studies of natural immunity to solid tumours in rats. Persistence of natural immunity throughout reproductive life, and absence of suppressor cells in infant rats. Immunology. 1980 Feb;39(2):187–194. [PMC free article] [PubMed] [Google Scholar]
- Bullen C. L., Willis A. T. Resistance of the breast-fed infant to gastroenteritis. Br Med J. 1971 Aug 7;3(5770):338–343. doi: 10.1136/bmj.3.5770.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Robins R. A., Brooks C. G., Baldwin R. W. Phenotype of rat natural killer cells defined by monoclonal antibodies marking rat lymphocyte subsets. Immunology. 1982 Jan;45(1):97–103. [PMC free article] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. The early appearance of specific cytotoxic T cells in murine gut mucosa. Clin Exp Immunol. 1980 Nov;42(2):273–279. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Jouanen E., Williams R. C., Jr T and B lymphocytes in human colostrum. Clin Immunol Immunopathol. 1974 Nov;3(2):248–255. doi: 10.1016/0090-1229(74)90011-7. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972 Dec;12(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hanson D. G. Ontogeny of orally induced tolerance to soluble proteins in mice. I. Priming and tolerance in newborns. J Immunol. 1981 Oct;127(4):1518–1524. [PubMed] [Google Scholar]
- Joel D. D., Hess M. W., Cottier H. Magnitude and pattern of thymic lymphocyte migration in neonatal mice. J Exp Med. 1972 Apr 1;135(4):907–923. doi: 10.1084/jem.135.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loop S. M., Bernstein I. D., Wright P. W. T cell synergy in the rat: serologic characterization of T cell subsets. J Immunol. 1980 Sep;125(3):1237–1239. [PubMed] [Google Scholar]
- Lyscom N., Brueton M. J. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982 Apr;45(4):775–783. [PMC free article] [PubMed] [Google Scholar]
- Manning L. S., Parmely M. J. Cellular determinants of mammary cell-mediated immunity in the rat. I. The migration of radioisotopically labeled T lymphocytes. J Immunol. 1980 Dec;125(6):2508–2514. [PubMed] [Google Scholar]
- Matthew D. J., Taylor B., Norman A. P., Turner M. W. Prevention of eczema. Lancet. 1977 Feb 12;1(8007):321–324. doi: 10.1016/s0140-6736(77)91131-x. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Intraepithelial lymphocyte count and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982 Aug;83(2):417–423. [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Orlic D., Gray B. A., Carmichael R. D., Baron P. Lymphocyte infiltration of gut epithelium in fetal and neonatal rabbits. Biol Neonate. 1981;40(1-2):91–98. doi: 10.1159/000241476. [DOI] [PubMed] [Google Scholar]
- Pilarski L. M. Ontogeny of cell-mediated immunity. I. Early development of alloantigen-specific cytotoxic T-cell precursors in postnatal mice. J Exp Med. 1977 Sep 1;146(3):887–892. doi: 10.1084/jem.146.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie E. R., Bass R., Meistrich M. L., Dennison D. K. Distribution of T lymphocyte subsets in human colostrum. J Immunol. 1982 Sep;129(3):1116–1119. [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Rose M. L., Parrott D. M., Bruce R. G. The accumulation of immunoblasts in extravascular tissues including mammary gland, peritoneal cavity, gut and skin. Immunology. 1978 Aug;35(2):415–423. [PMC free article] [PubMed] [Google Scholar]
- Savilahti E. Immunoglobulin-containing cells in the intestinal mucosa and immunoglobulins in the intestinal juice in children. Clin Exp Immunol. 1972 Jul;11(3):415–425. [PMC free article] [PubMed] [Google Scholar]
- Seelig L. L., Jr, Billingham R. E. Capacity of "transplanted" lymphocytes to traverse the intestinal epithelium of adult rats. Transplantation. 1981 Oct;32(4):308–314. doi: 10.1097/00007890-198110000-00010. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Befus A. D., Clark D. A., Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982 Jun 1;155(6):1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Luini W., Soldateschi D., Boraschi D. Natural killer activity of gut mucosal lymphoid cells in mice. Eur J Immunol. 1981 Nov;11(11):919–922. doi: 10.1002/eji.1830111112. [DOI] [PubMed] [Google Scholar]
- Thomas J., Anderson N. V. Interepithelial lymphocytes in the small intestinal mucosa of conventionally reared dogs. Am J Vet Res. 1982 Feb;43(2):200–203. [PubMed] [Google Scholar]
- Webb M., Mason D. W., Williams A. F. Inhibition of mixed lymphocyte response by monoclonal antibody specific for a rat T lymphocyte subset. Nature. 1979 Dec 20;282(5741):841–843. doi: 10.1038/282841a0. [DOI] [PubMed] [Google Scholar]


