Abstract
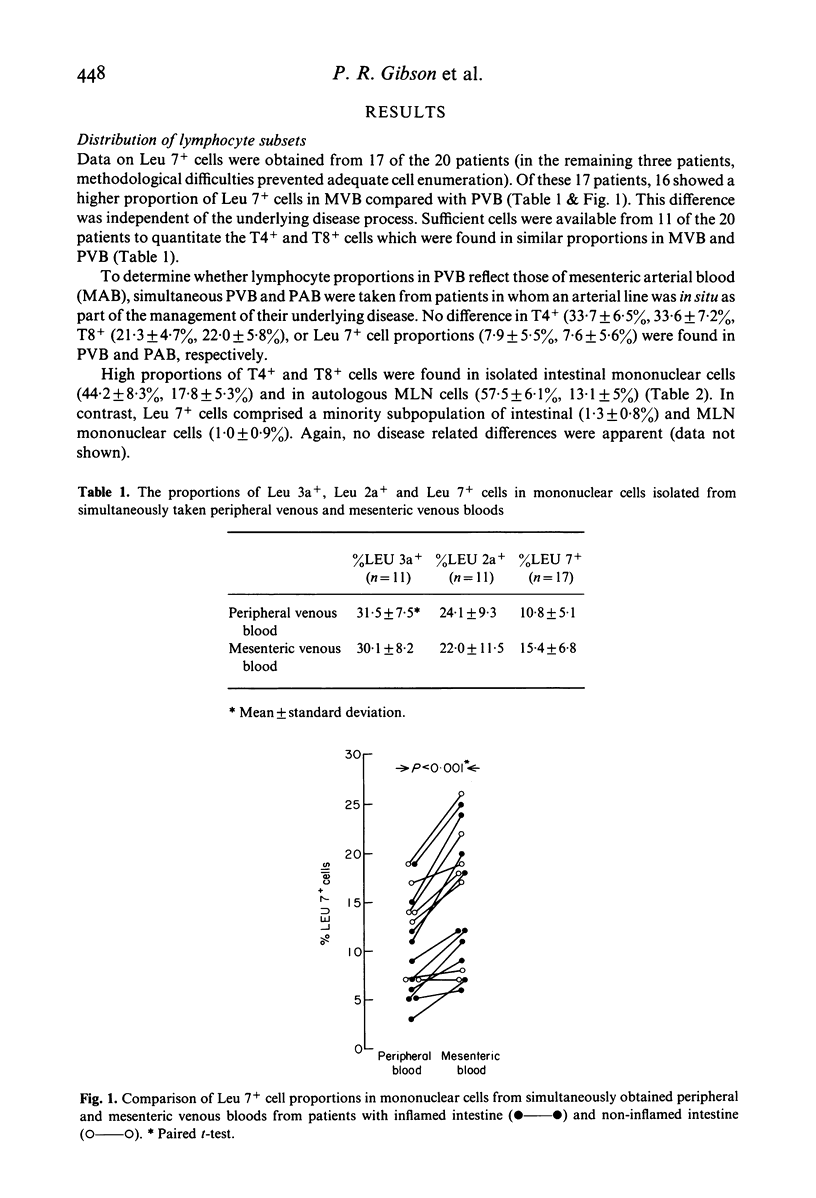
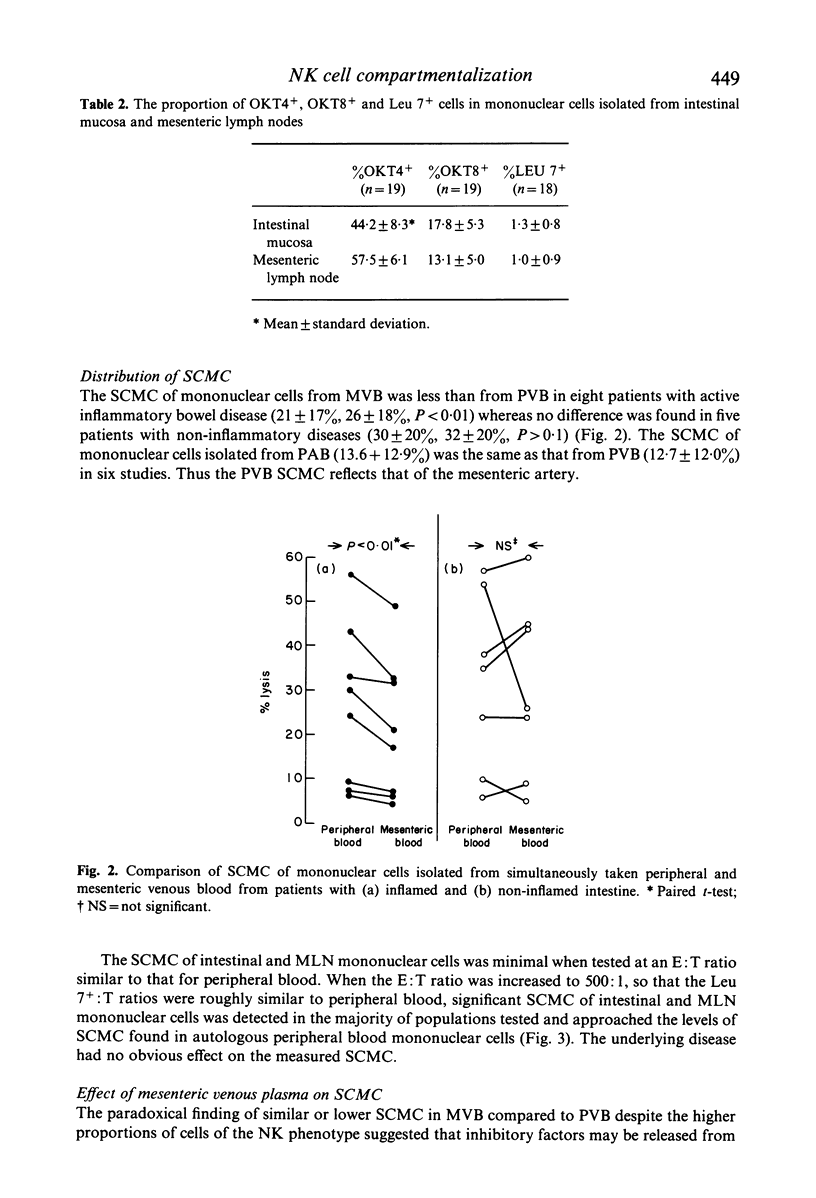
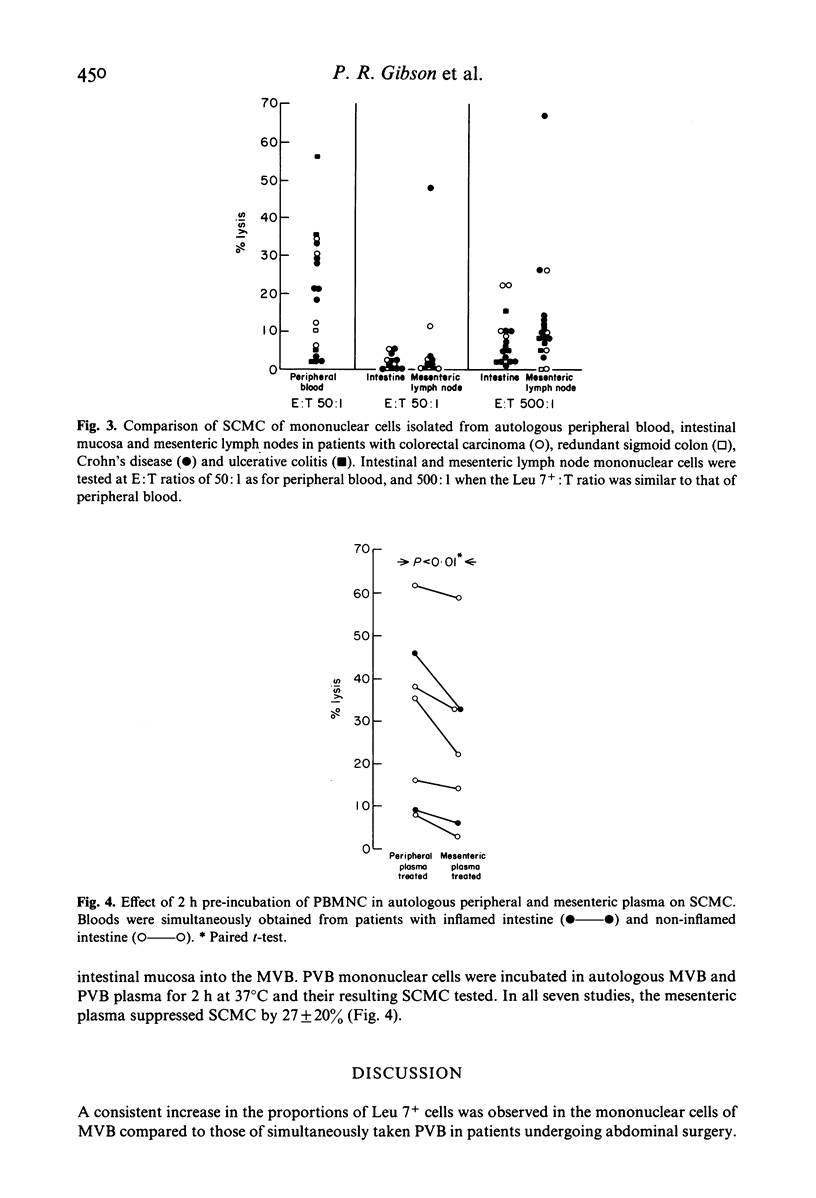
The proportions of T cell subsets and Leu 7+ cells and the spontaneous cell-mediated cytotoxicity (SCMC) of isolated mononuclear cells have been determined across the mesenteric vascular bed and along the intestinal mucosal-mesenteric lymph node (MLN) axis in patients undergoing abdominal surgery. Whereas the proportion of T4+ and T8+ cells were similar in simultaneously taken PVB and mesenteric venous blood (MVB), the proportion of Leu 7+ cells was higher in MVB in 16 of 17 studies (15.4 +/- 6.8%, 10.8 +/- 5.1%). Additional studies showed that the proportions of lymphocyte subsets in peripheral arterial blood are the same as those in PVB. Thus, an enrichment of Leu 7+ cells occurs across the mesenteric vascular bed. Isolated intestinal and MLN mononuclear cells contained similarly high proportions of T4+ and T8+ cells as in PVB but Leu 7+ cells made up a minority subpopulation in intestinal (1.3 +/- 0.8%) and MLN mononuclear cells (1.0 +/- 0.9%). The SCMC of intestinal and MLN mononuclear cells was low and paralleled the proportion of Leu 7+ cells. Despite the higher proportions of Leu 7+ cells in MVB, the SCMC was less than that of PVB in eight patients with inflamed intestine and not significantly different from PVB in seven patients with normal intestines. These paradoxical findings were at least in part due to inhibitory factors in mesenteric plasma. In conclusion, NK cells appear to be largely confined within the vascular system and the enrichment of Leu 7+ cells across the mesenteric vascular bed suggests that this compartmentalization may be due to differences in the traffic of lymphocyte subpopulations through the intestinal mucosa and MLN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Glickman E., Evans R. L. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981 Jul 1;154(1):193–198. doi: 10.1084/jem.154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J. A possible route for lymphocyte migration into diseased tissues. J Clin Pathol. 1983 Feb;36(2):161–166. doi: 10.1136/jcp.36.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959 Apr 23;146(1):54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- McConnell I. Immunology. Roving lymphocytes. Nature. 1983 Jul 7;304(5921):17–17. doi: 10.1038/304017a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Jewell D. P. T lymphocyte subsets in inflammatory bowel disease: peripheral blood. Gut. 1983 Feb;24(2):99–105. doi: 10.1136/gut.24.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., McIntosh G. H., Morris B. The migration of cells through chronically inflamed tissues. J Pathol. 1970 Jan;100(1):21–29. doi: 10.1002/path.1711000104. [DOI] [PubMed] [Google Scholar]
- Zöller M., Bellgrau D., Axberg I., Wigzell H. Natural killer cells do not belong to the recirculating lymphocyte population. Scand J Immunol. 1982 Feb;15(2):159–167. doi: 10.1111/j.1365-3083.1982.tb00634.x. [DOI] [PubMed] [Google Scholar]


