Abstract
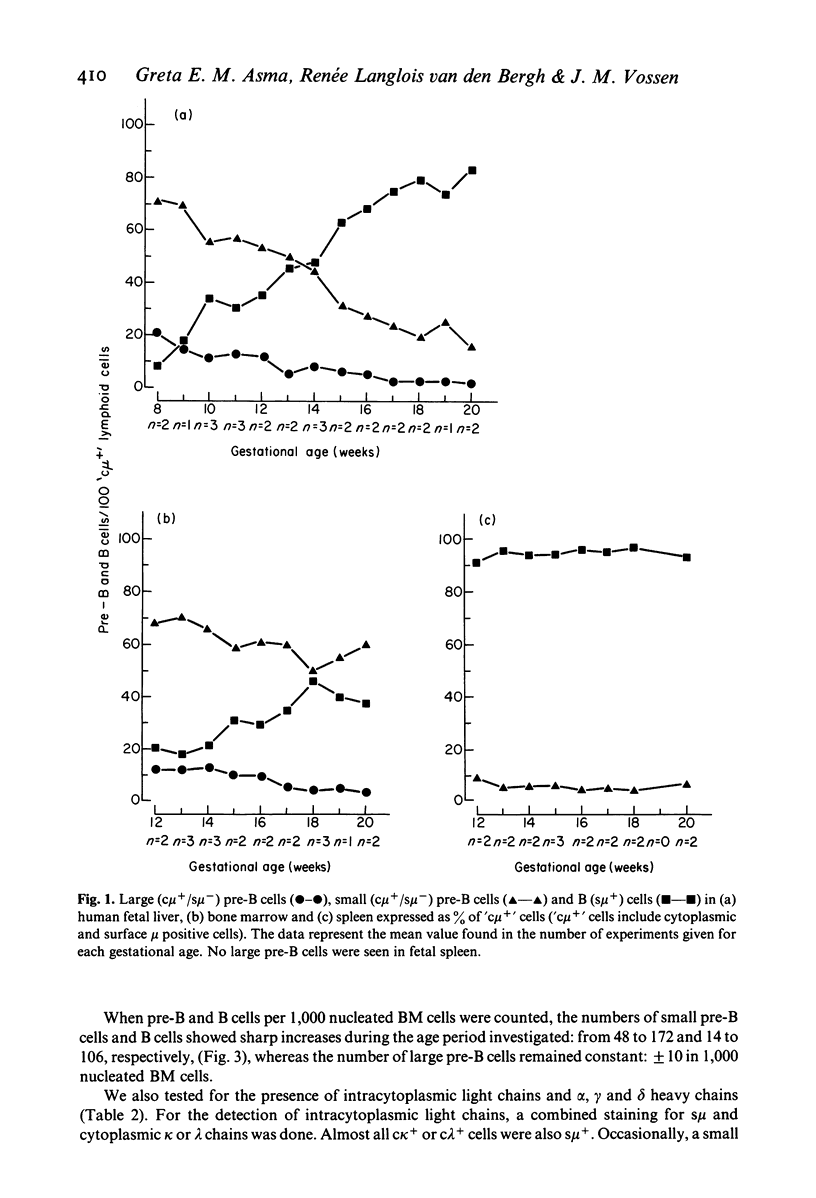
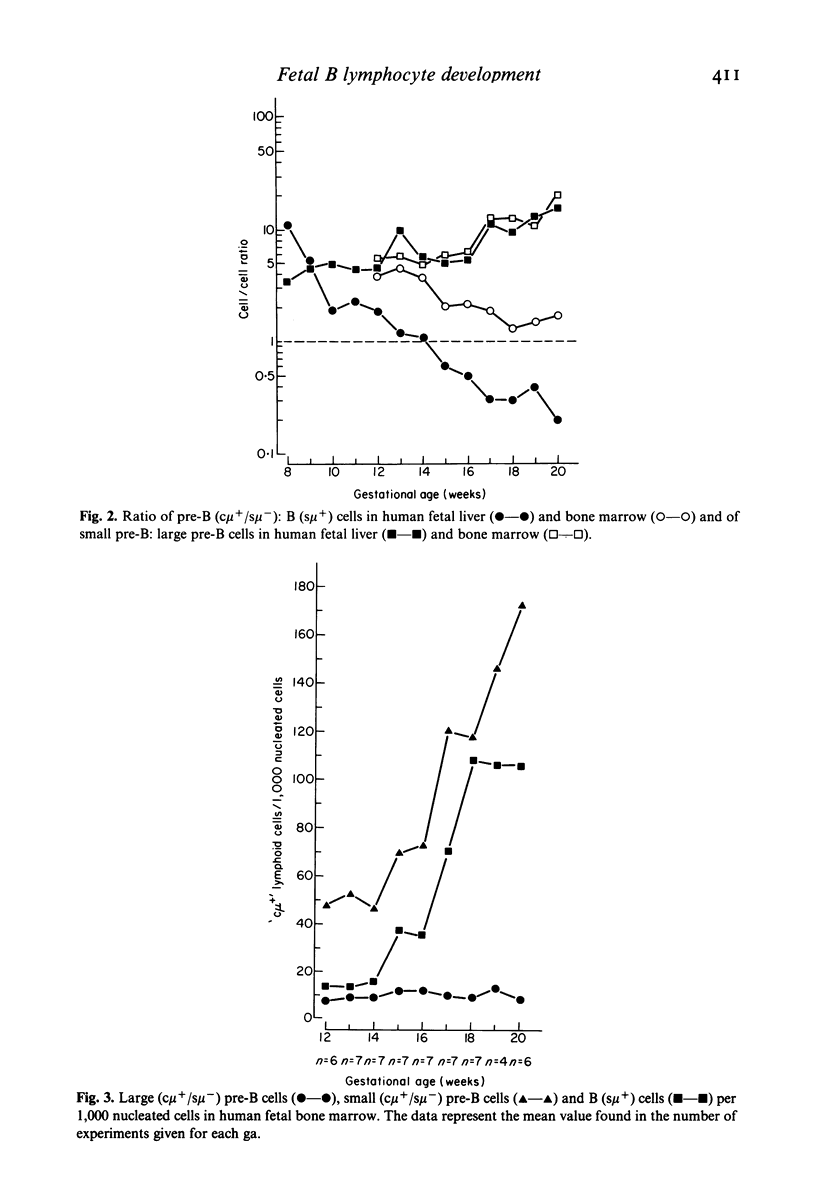
Cell suspensions from human fetal liver, bone marrow and spleen were systematically studied at between the fetal ages of 8 and 20 weeks by the direct immunofluorescence technique for the presence of pre-B and B cells. Pre-B cells were characterized as lymphoid cells containing cytoplasmic mu heavy chains but lacking surface IgM. Based on their size and morphological appearance, these cells were subdivided into large and small pre-B cells. In the livers of 8 week old fetuses, more than 90% of the total pre-B plus B cell population consisted of pre-B cells; the relative number of liver pre-B cells gradually decreased with increasing gestational age and, after the 14th week, B cells outnumbered pre-B cells. At 20 weeks, the ratio of pre-B to B cells was only 0.25. In contrast, the number of pre-B cells in fetal bone marrow (12-20 weeks) was always greater than that of the B cells. Large and small pre-B cells were present in the liver and bone marrow. Small pre-B cells outnumbered the large ones in both organs and with increasing gestational age the ratio of small to large pre-B cells increased four-fold. In fetal spleen (12-20 weeks), no large pre-B cells were seen and the small ones comprised only a minor fraction of the total B-cell population. It can be concluded from these data that during early human fetal life the liver is an important site of pre-B cell production. From 12 weeks onwards, this function is gradually taken over by the bone marrow. During the second half of pregnancy, pre-B cell production in fetal liver becomes very much less as compared with the bone marrow. No generation of pre-B cells takes place in the fetal spleen, but a certain amount of maturation of cells of the B cell line may take place in this organ.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asma G. E., Van den Bergh R. L., Vossen J. M. Use of monoclonal antibodies in a study of the development of T lymphocytes in the human fetus. Clin Exp Immunol. 1983 Aug;53(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Bolger P. M., Eisner G. M., Ramwell P. W., Slotkoff L. M. Effect of prostaglandin synthesis on renal function and renin in the dog. Nature. 1976 Jan 22;259(5540):244–245. doi: 10.1038/259244a0. [DOI] [PubMed] [Google Scholar]
- Burrows P. D., Kearney J. F., Lawton A. R., Cooper M. D. Pre-B cells: bone marrow persistence in anti-mu-suppressed mice, conversion to B lymphocytes, and recovery after destruction by cyclophosphamide. J Immunol. 1978 May;120(5):1526–1531. [PubMed] [Google Scholar]
- Cooper M. D. Pre-B cells; normal and abnormal development. J Clin Immunol. 1981 Apr;1(2):81–89. doi: 10.1007/BF00915383. [DOI] [PubMed] [Google Scholar]
- Dhaliwal H. S., Ling N. R., Bishop S., Chapel H. Expression of immunoglobin G on blood lymphocytes in chronic lymphocytic leukaemia. Clin Exp Immunol. 1978 Feb;31(2):226–236. [PMC free article] [PubMed] [Google Scholar]
- Freitas A. A., Rocha B., Forni L., Coutinho A. Population dynamics of B lymphocytes and their precursors: demonstration of high turnover in the central and peripheral lymphoid organs. J Immunol. 1982 Jan;128(1):54–60. [PubMed] [Google Scholar]
- Fulop G., Gordon J., Osmond D. G. Regulation of lymphocyte production in the bone marrow. I. Turnover of small lymphocytes in mice depleted of B lymphocytes by treatment with anti-IgM antibodies. J Immunol. 1983 Feb;130(2):644–648. [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Simons M. A., Lawton A. R., Mage R. G., Cooper M. D. Pre-B and B cells in rabbits. Ontogeny and allelic exclusion of kappa light chain genes. J Exp Med. 1978 Nov 1;148(5):1367–1377. doi: 10.1084/jem.148.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Klein F. An immunofluorescence procedure for the detection of intracellular immunoglobulins. Clin Exp Immunol. 1969 Apr;4(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Kamps W. A., Cooper M. D. Microenvironmental studies of pre-B and B cell development in human and mouse fetuses. J Immunol. 1982 Aug;129(2):526–531. [PubMed] [Google Scholar]
- Kubagawa H., Gathings W. E., Levitt D., Kearney J. F., Cooper M. D. Immunoglobulin isotype expression of normal pre-B cells as determined by immunofluorescence. J Clin Immunol. 1982 Oct;2(4):264–269. doi: 10.1007/BF00915065. [DOI] [PubMed] [Google Scholar]
- Landreth K. S., Rosse C., Clagett J. Myelogenous production and maturation of B lymphocytes in the mouse. J Immunol. 1981 Nov;127(5):2027–2034. [PubMed] [Google Scholar]
- Melchers F., Von Boehmer H., Phillips R. A. B-lymphocyte subpopulations in the mouse. Organ distribution and ontogeny of immunoglobulin-synthesizing and of mitogen-sensitive cells. Transplant Rev. 1975;25:26–58. doi: 10.1111/j.1600-065x.1975.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Owen J. J., Raff M. C., Cooper M. D. Studies on the generation of B lymphocytes in the mouse embryo. Eur J Immunol. 1976 Jul;5(7):468–473. doi: 10.1002/eji.1830050708. [DOI] [PubMed] [Google Scholar]
- Owen J. J., Wright D. E., Habu S., Raff M. C., Cooper M. D. Studies on the generation of B lymphocytes in fetal liver and bone marrow. J Immunol. 1977 Jun;118(6):2067–2072. [PubMed] [Google Scholar]
- Paige C. J. Surface immunoglobulin-negative B-cell precursors detected by formation of antibody-secreting colonies in agar. Nature. 1983 Apr 21;302(5910):711–713. doi: 10.1038/302711a0. [DOI] [PubMed] [Google Scholar]
- Pearl E. R., Vogler L. B., Okos A. J., Crist W. M., Lawton A. R., 3rd, Cooper M. D. B lymphocyte precursors in human bone marrow: an analysis of normal individuals and patients with antibody-deficiency states. J Immunol. 1978 Apr;120(4):1169–1175. [PubMed] [Google Scholar]
- Phillips R. A., Melchers F. Appearance of functional lymphocytes in fetal liver. J Immunol. 1976 Oct;117(4):1099–1103. [PubMed] [Google Scholar]
- Rosenberg Y. J., Parish C. R. Ontogeny of the antibody-forming cell line in mice. IV. Appearance of cells bearing Fc receptors, complement receptors, and surface immunoglobulin. J Immunol. 1977 Feb;118(2):612–617. [PubMed] [Google Scholar]
- Touraine J. L. Bone-marrow and fetal-liver transplantation in immunodeficiencies and inborn errors of metabolism: lack of significant restriction of T-cell function in long-term chimeras despite HLA-mismatch. Immunol Rev. 1983;71:103–121. doi: 10.1111/j.1600-065x.1983.tb01070.x. [DOI] [PubMed] [Google Scholar]
- de Gast G. C., Platts-Mills T. A. Functional studies on lymphocytes in adult human bone marrow. II. Isolated surface IgM-positive cells. J Immunol. 1979 Jan;122(1):285–290. [PubMed] [Google Scholar]


