Abstract
The generation and maintenance of immunological memory requires the activation, expansion, and persistent proliferation of antigen-specific T cells. Recent work suggests that IL-15 may be important for this process. Surprisingly, we now find that expression of the high-affinity receptor for IL-15, IL-15Rα, on T cells is dispensable for the generation or maintenance of memory CD8+ T cells. By contrast, IL-15Rα expression on cells other than T cells is absolutely critical for this function. These findings may be related to IL-15Rα's ability to present IL-15 in trans to low-affinity IL-15Rβ/γc receptors on memory CD8+ T cells. These unexpected results provide insights into how IL-15Rα supports memory CD8+ T cells.
Memory CD8+ T cells are critical mediators of immunity to intracellular pathogens. The signals that support homeostasis of memory CD8+ T cells have only recently begun to be elucidated. Unlike their naïve counterparts, memory CD8+ T cells do not require interaction with cognate MHC (1). Rather, cytokines that use the common γ (γc) chain for receptor signaling appear to play a central role in the support of memory CD8+ T cells. Two such cytokines, IL-7 and IL-15, have been shown to regulate memory CD8+ T cell homeostasis. However, whereas IL-7 primarily supports proliferation of both naïve and memory CD8+ T cells in lymphopenic states, IL-15 specifically regulates the survival and proliferation of memory CD8+ T cells under normal physiological conditions.
IL-15 is a four-α-helix bundle family cytokine both functionally and structurally similar to IL-2 (2, 3). The heterotrimeric IL-15 receptor (IL-15R) consists of IL-15Rα, which uniquely binds IL-15, as well as IL-2Rβ and γc (4, 5). IL-15Rα alone binds IL-15 with a Kd of ≈1 × 10−11 M. Consistent with IL-15's functional homology to IL-2, IL-15R shares IL-2Rβ and γc subunits with IL-2R, and these subunits mediate signal-transduction events after IL-2 or IL-15 binding. Whereas IL-2Rβ and γc are able to bind IL-15 without IL-15Rα, the similar phenotypes of IL-15−/− and IL-15Rα−/− mice suggest that IL-15Rα is required for IL-15 signals in vivo.
Several lines of evidence suggest that IL-15 selectively supports the survival and proliferation of both memory and memory phenotype (CD44Hi IL-2RβHi) CD8+ T cells. First, memory phenotype cells express high levels of IL-2Rβ, a key component of the IL-15R complex, and in vivo administration of anti-IL-2Rβ-specific, but not anti-IL-2Rα-specific, antibodies blocks the basal proliferation of these cells (6). Second, administration of recombinant IL-15 selectively stimulates the proliferation of memory phenotype CD8+ T cells both in vivo and in vitro (7). Third, both IL-15−/− and IL-15Rα−/− mice have severely reduced numbers of memory phenotype cells, whereas mice that overexpress a readily secreted form of IL-15 possess increased numbers of these cells (8–10). Finally, recent studies using IL-15−/− or IL-15Rα−/− mice suggest that antigen-experienced CD8+ memory T cells require both IL-15 and IL-15Rα for basal proliferation and long-term survival (11–14). Thus, it appears that IL-15 is critical for the proliferation and survival of both memory and memory phenotype CD8+ T cells in vivo.
As IL-15Rα alone binds soluble IL-15 with high affinity, the studies above reinforce the notion that soluble IL-15 binds to IL-15Rα receptors on memory CD8+ T cells to stimulate their survival and/or proliferation. However, several observations suggest that this straightforward explanation may not adequately explain the mechanism by which IL-15 supports memory CD8+ T cells. First, although the IL-2Rβ and γc receptor chains common to both IL-2 and IL-15 heterotrimeric receptors are thought to initiate cytoplasmic signal-transduction pathways from these receptors, the phenotypes of IL-2−/− and IL-2Rα−/− mice are dramatically divergent from the phenotypes of IL-15−/− and IL-15Rα−/− mice (9, 10, 15, 16). It is unclear as to how IL-15 receptor-initiated signals would be distinguished from IL-2 receptor-initiated signals on the same T cells. Second, unlike IL-2Rα, IL-15Rα is expressed on multiple cell types, including nonlymphoid and even nonhematopoietic cells. Recent data from our laboratory suggest that IL-15Rα signals on radiation-sensitive hematopoietic cells other than T cells support poly-(inosinic acid)⋅poly(cytidylic acid) [poly(I⋅C)]-induced bystander proliferation responses of memory phenotype CD8+ T cells (17). Hence, T cell-independent IL-15Rα signals may also be important for supporting antigen-experienced memory CD8+ T cells. To directly evaluate the potential roles of IL-15Rα signals in supporting antigen-specific CD8+ T cells, we have studied the functions of both T cell-dependent and T cell-independent IL-15Rα signals in regulating antigen-specific responses of OT-1+ CD8+ T cells.
Materials and Methods
Mice.
The generation and preliminary characterization of IL-15Ra−/− mice were described (10). All IL-15Ra−/− mice were backcrossed to Ly5.1+ C57BL/6J or congenic Ly5.2+ C57BL/6J/SJL mice (The Jackson Laboratory) for eight or nine generations. OT-1+ recombinase-activating gene (RAG)-1−/− mice on a C57BL/6J background were obtained from Kristen Hogquist and Matthew Messcher (University of Minnesota, Minneapolis). IL-15Rα−/− OT-1+ RAG-1−/− mice were generated by interbreeding in our facility. Bone marrow chimeras were produced as previously described, using 950 rad of total body γ-irradiation, except that some bone marrow chimera received a split dose of 1,100 rad (550 rad delivered twice, separated by 3 h) after 4 months to ensure that radiation-sensitive cells were eliminated before immunization (17). All mice were housed and bred in specific pathogen-free facilities according to University of Chicago Institutional Animal Care and Use Committees guidelines.
Adoptive Transfers and Immunization.
OT-1+ RAG-1−/− or IL-15Rα−/− OT-1+ RAG-1−/− cells were isolated from lymph nodes and consisted of >95% CD8+ Vα2+ CD44Lo IL-2RβLo cells. For carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling, cells were washed in PBS and then were incubated at room temperature with 5 μM CFSE (Molecular Probes) in PBS. Labeling was quenched with an equal volume of FCS, and the cells were washed before adoptive transfer. Two days before immunization, mice received 2–4 × 106 OT-1+ RAG-1−/− CD8+ T cells through either the lateral tail vein or the retro-orbital vein. Mice were immunized i.p. with 250 μl of PBS, 2.5 mg of ovalbumin (OVA) (Sigma-Aldrich), 150 μg of poly(I⋅C) (Pharmacia Biotech), or both OVA and poly(I⋅C). For adoptive transfers of memory OT-1+ cells, splenocytes and lymph node T cells were harvested from immune mice >40 days after immunization and transferred intravenously into IL-15Rα+/− or IL-15Rα−/− mice. In some cases, cells were labeled with CFSE before transfer.
Cell Preparation.
Peripheral blood lymphocytes (PBLs) were obtained by withdrawing ≈100 μl of blood through retro-orbital vein puncture. RBC lysis and preparations of single-cell suspensions from lymph nodes and spleens were preformed as described (10). Lamina propria lymphocytes were isolated as described (18). Hepatic lymphocytes were isolated from livers that were homogenized through nylon mesh and washed twice in PBS with 5% FCS. Cells were then resuspended in 44% Percoll (Sigma-Aldrich), underlaid with 67% Percoll, and centrifuged for 20 min at 700 × g. Cells at the interface were collected, washed, and counted.
Antibodies, Staining, and Flow Cytometric Analysis.
Fluorescently labeled antibodies and streptavidin secondary reagents were purchased from BD PharMingen. H2Kb-Ig dimer (BD PharMingen) was loaded with either SIINFEKL (henceforth referred to as H2Kb-OVA) or SIYRYYGL (negative control) peptides as per the manufacturer's instructions. Dimer staining of cells was performed as per manufacturer's recommendations. Cells were preincubated with FcBlock (BD PharMingen) before staining. Cells were analyzed with a FACSCalibur flow cytometer equipped with cellquest software (Becton Dickinson). For ex vivo IFN-γ staining, cells were incubated in vitro in complete RPMI medium 1640 with 1 μl/ml Golgistop (BD PharMingen) with or without 40 μg/ml SIINFEKL peptide for 4–5 h at 37°C. Cells were then collected, fixed, permeabilized, and stained by using a Cytofix/Cytoperm kit according to manufacturer's instructions (BD PharMingen).
Trans Presentation of IL-15 to IL-15Rα−/− T Cells.
IL-15Rα-mediated trans presentation of IL-15 to memory CD8+ T cells was evaluated by using recombinant murine (rm)IL-15Rα/Fc chimeric protein (R & D Systems). Tissue culture plates were coated with 3 μg/ml rmIL-15Rα/Fc for 1 h at 37°C and then blocked with PBS containing 5% serum for an equivalent length of time. Various amounts of rmIL-15 (PeproTech, Rocky Hill, NJ) were then added, and the plates were incubated for 1 h at 37°C. Plates were then washed three times with PBS containing 5% serum. Lymph node T cells were purified to >95% purity from immune mice by magnetic-bead depletion of B cells by using anti-Ig beads (Dynal, Great Neck, NY), and plated on these coated wells in RPMI medium 1640. After 4 days, T cells were collected, counted, and assessed for viability by propidium iodide staining and light-scatter profile.
Results
Poly(I⋅C) Is an Effective Adjuvant for OT-1+ CD8+ T Cell Responses.
The magnitude of the primary expansion and differentiation of antigen-specific T cells influences the subsequent generation of memory T cells. As the magnitude of the primary expansion of CD8+ T cells is related to the precursor frequency of antigen-specific T cells, and as IL-15Rα−/− (and IL-15−/−) mice possess lower numbers of CD8+ T cells than do normal mice, we bred IL-15Rα−/− mice to a C57BL/6J background and used an adoptive transfer system in which the numbers of antigen-specific cells could be defined precisely before and during immune responses (19, 20). As memory CD8+ T cells mediate immunity against viral pathogens, and as poly(I⋅C) is an innate immune stimulant that mimics viral RNA, we used poly(I⋅C) as an adjuvant to augment primary CD8+ T cell responses and generate memory responses. To determine whether poly(I⋅C) would augment the expansion of OT-1+ CD8+ T cells (which recognize the SIINFEKL peptide of OVA in the context of H-2Kb), cells from Ly5.1+ OT-1+ RAG-1−/− mice were adoptively transferred into Ly5.2+ congenic mice. Small numbers of OT-1+ CD8+ T cells were recovered 6 days after adoptive transfer into nonimmunized congenic mice, and these cells retained a naïve phenotype (CD44Lo, IL-2RβLo; data not shown). Immunization with OVA 2 days after adoptive transfer increased the number of H-2Kb-OVA-reactive Ly5.1+ OT-1+ CD8+ T cells recovered in spleen and lymph nodes 6 days after transfer and caused these OT-1+ CD8+ T cells to express elevated levels of the activation markers CD44 and IL-2Rβ (see Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). Importantly, immunization with poly(I⋅C) plus OVA further increased the yield of OT-1+ CD8+ T cells in these compartments ≈10-fold, demonstrating that poly(I⋅C) is an effective adjuvant in enhancing primary CD8+ T cell responses (see Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). No expansion or induction of CD44 expression on OT-1+ T CD8+ cells was seen in mice immunized with poly(I⋅C) without OVA, demonstrating that cognate antigen is required for expansion of these cells (data not shown). Finally, these experiments demonstrate that poly(I⋅C) is an effective adjuvant for primary CD8+ T cell responses.
In addition to augmenting primary immune responses to cognate antigens, adjuvants also induce effective development of a stable pool of memory cells. Thus, we assessed the generation and persistence of memory OT-1+ CD8+ T cells in immunized mice. The number of OT-1+ CD8+ cells recovered from OVA-immunized mice peaked at day 4 after immunization and fell dramatically until day 11, and eventually these cells were undetectable above background (Fig. 7B). The number of OT-1+ CD8+ T cells recovered from poly(I⋅C) plus OVA-immunized mice also peaked 4 days after immunization, albeit at much higher levels, fell dramatically until day 20, and then stabilized thereafter (Fig. 1, and Fig. 8, which is published as supporting information on the PNAS web site). OT-1+ CD8+ T cells harvested from the latter mice >50 days after immunization expressed high levels of CD44 (Fig. 8). These cells also expressed IFN-γ within 4 h of ex vivo exposure to SIINFEKL peptide, confirming that they were functional memory OT-1+ CD8+ T cells (Fig. 8B). Thus, immunization with poly(I⋅C) and OVA induces the generation and maintenance of functional memory OT-1+ CD8+ T cells.
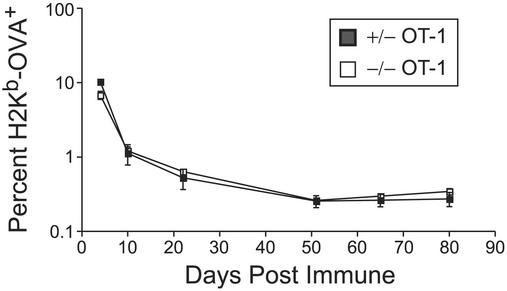
Figure 1.
IL-15Rα expression on CD8+ T cells is dispensable for CD8+ T cell memory. Similar percentages of IL-15Rα+/− (■) and IL-15Rα−/− (□) OT-1+ RAG−/− CD8+ T cells at memory time points in the PBL. Data represent the mean of four mice per time point ± SEM.
IL-15Rα Expression on Memory OT-1+ CD8+ T Cells Is Not Required for Their Generation or Maintenance.
Prior studies suggested that soluble IL-15 supports the proliferation of memory phenotype and memory CD8+ T cells by binding to IL-15 receptors on these cells (7, 13, 14). To directly determine whether IL-15Rα expression on CD8+ T cells provides essential signals for the generation or maintenance of memory CD8+ T cells, IL-15Rα+/− OT-1+, or IL-15Rα−/− OT-1+ CD8+ T cells were adoptively transferred into normal mice. The expansion of these cells was then examined after immunization with poly(I⋅C) plus OVA. Serial PBL analyses from immunized mice bearing IL-15Rα+/− OT-1+ or IL-15Rα−/− OT-1+ CD8+ T cells revealed that nearly identical numbers of IL-15Rα+/− and IL-15Rα−/− CD44Hi, IL-2RβHi OT-1+ CD8+ T cells were obtained from PBLs 10–80 days postimmunization (Fig. 1). Both IL-15Rα+/− OT-1+ and IL-15Rα−/− OT-1+ CD8+ T cells rapidly expressed IFN-γ in response to in vitro challenge with the cognate SIINFEKL peptide (data not shown). Thus, despite being unable to produce the heterotrimeric high-affinity IL-15 receptor complex, naïve IL-15Rα−/− OT-1+ CD8+ T cells differentiated into functional memory CD8+ T cells in normal mice. Hence, IL-15Rα expression on CD8+ T cells is dispensable for memory CD8+ T cells.
T Cell-Independent IL-15Rα Signals Support Memory OT-1+ CD8+ T Cell Responses.
In contrast to the IL-2Rα, IL-15Rα is expressed on multiple cell types, including on nonhematopoietic cells. Recent work from our laboratory suggests that IL-15Rα signals on cells other than T cells regulate CD8+ T cell bystander proliferation responses to poly(I⋅C) (17). Accordingly, we investigated whether T cell-independent IL-15Rα signals were important for antigen-specific memory CD8+ T cells by asking whether IL-15Rα−/− mice could support immune responses of IL-15Rα competent OT-1+ CD8+ T cells to poly(I⋅C) plus OVA. OT-1+ CD8+ T cells were adoptively transferred into either IL-15Rα+/− or IL-15Rα−/− congenic mice, and these mice were immunized with OVA with or without poly(I⋅C). Quantitation of OT-1+ CD8+ T cells in PBL, spleen, and lymph nodes from nonimmunized IL-15Rα+/− and IL-15Rα−/− recipient mice 6 days after transfer revealed that similar numbers of these cells (as indicated by percentages of PBLs) were present in both mouse strains (Fig. 2). Thus, IL-15Rα+/− and IL-15Rα−/− mice support the short-term maintenance of naïve transgenic OT-1+ CD8+ T cells equally well. The percentages of OT-1+ CD8+ cells observed 4 days after immunization with either OVA alone or poly(I⋅C) plus OVA were also identical in IL-15Rα+/− mice when compared with IL-15Rα−/− mice, demonstrating that IL-15Rα−/− mice support normal primary expansion of OT-1+ CD8+ T cells (Fig. 2). Interestingly, the critical role of T cell-independent IL-15Rα signals in supporting poly(I⋅C)-induced bystander proliferation does not seem to be important for the poly(I⋅C)-mediated augmentation of antigen-stimulated OT-1+ CD8+ T cells during primary responses (17). Nevertheless, our data clearly demonstrate that OT-1+ CD8+ T cells proliferate, expand, and acquire effector function in primary responses to poly(I⋅C) and OVA in both IL-15Rα+/− and IL-15Rα−/− mice.
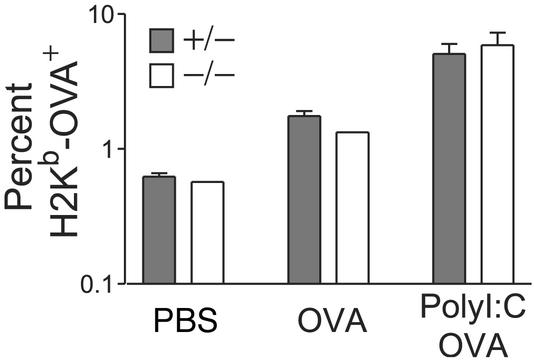
Figure 2.
Naïve IL-15Rα-competent CD8+ T cells show normal short-term maintenance and initial expansion in response to either OVA or OVA plus poly(I⋅C) in both IL-15Rα+/− and IL-15Rα−/− mice. There were similar percentages of H2Kb-OVA+ CD8+ cells in the peripheral lymph nodes (PLNs) 4 days after immunization in both IL-15Rα+/− and IL-15Rα−/− mice. Plots are representative of at least two mice per condition. Data represent mean ± SEM.
The normal primary response of IL-15Rα-competent OT-1+ CD8+ T cells in IL-15Rα+/− and IL-15Rα−/− mice allows a direct comparison of the role of IL-15Rα in memory OT-1+ CD8+ T cell generation and maintenance. To determine whether IL-15Rα−/− mice can support the generation and maintenance of OT-1+ CD8+ memory T cells, longitudinal analyses of PBLs from immunized mice were performed. Whereas the percentage of memory OT-1+ CD8+ T cells persisted at relatively stable levels in IL-15Rα+/− mice between 20 and 130 days postimmunization, the percentage of these cells declined precipitously between days 4 and 30 postimmunization in IL-15Rα−/− mice, such that they were essentially undetectable above background levels after day 30 (Fig. 3A). These percentages understate the differences in absolute numbers of OT-1+ CD8+ T cells between IL-15Rα+/− and IL-15Rα−/− mice, because IL-15Rα−/− mice are lymphopenic (10). Thus, IL-15Rα expression on cells other than T cells is critical for the generation or maintenance of antigen-experienced memory OT-1+ CD8+ T cells, despite normal primary responses of these cells.
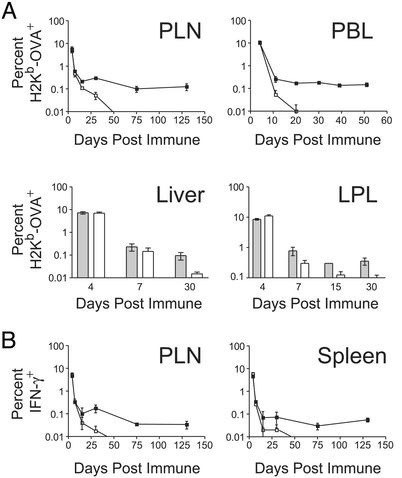
Figure 3.
IL-15Rα−/− mice fail to support IL-15Rα-competent memory CD8+ T cells, despite normal initial responses. (A) Percentages of H2Kb-OVA+ in the PLN, PBL, liver, and lamina propria lymphocytes (LPLs) of IL-15Rα+/− (filled squares or bars) or IL-15Rα−/− mice (open squares or bars) at various time points following immunization with OVA and poly(I⋅C). (B) Percentages of IFN-γ+ CD8+ T cells are shown in the PLN and spleen of IL-15Rα+/− (filled squares) or IL-15Rα−/− mice (open squares) at various time points after immunization with OVA and poly(I⋅C). Data represent mean ± SEM and all data points are reflective of at least two mice, with most reflecting three or more mice.
Memory T cells preferentially recirculate through tertiary lymphoid tissues because of the selective expression of adhesion molecules favoring migration to these tissues (21, 22). Recent studies suggest that IL-15 may be important in the differentiation of distinct subsets of memory CD8+ T cells (23). Thus, the loss of memory OT-1+ CD8+ T cells from the peripheral blood of IL-15Rα−/− mice may be because of differential homing of these cells. We examined the number of memory OT-1+ CD8+ T cells in secondary and tertiary lymphoid tissues from immunized IL-15Rα+/− and IL-15Rα−/− mice. Whereas stable populations of OT-1+ memory cells were recovered from spleens, peripheral lymph nodes, livers, and intestinal lamina propria up to 130 days postimmunization, no memory cells could be detected in any of these compartments in IL-15Rα−/− mice (Fig. 3). Thus, T cell-independent IL-15Rα signals are critical for the maintenance, rather than the homing, of antigen-experienced memory OT-1+ CD8+ T cells.
T Cell-Independent IL-15Rα Signals Support the Proliferation of Memory OT-1+ CD8+ T Cells.
The dramatic loss of memory OT-1+ CD8+ T cells in immunized IL-15Rα−/− mice may be because of the failure to generate and/or maintain these memory cells. To directly examine whether IL-15Rα signals are required for maintaining normal memory CD8+ T cells, we generated IL-15Rα+/− memory CD8+ T cells by transferring Ly5.1+ OT-1+ CD8+ T cells into congenic Ly5.2+ normal mice and immunized these mice with poly(I⋅C) plus OVA. At least 6 weeks after immunization, lymph node T cells from these Ly5.2+ mice, including IL-15Rα+/− Ly5.1+ memory OT-1+ CD8+ T cells, were adoptively transferred into either IL-15Rα+/− or IL-15Rα−/− congenic Ly5.2+ mice. Analyses of tissues from these latter mice 100 days later revealed that markedly fewer Ly5.1+ IFN-γ+ memory OT-1+ CD8+ T cells were present in spleens and lymph nodes of IL-15Rα−/− mice than in IL-15Rα+/− mice (Fig. 4A). Thus, IL-15Rα−/− mice are unable to support normal OT-1+ memory CD8+ T cells.
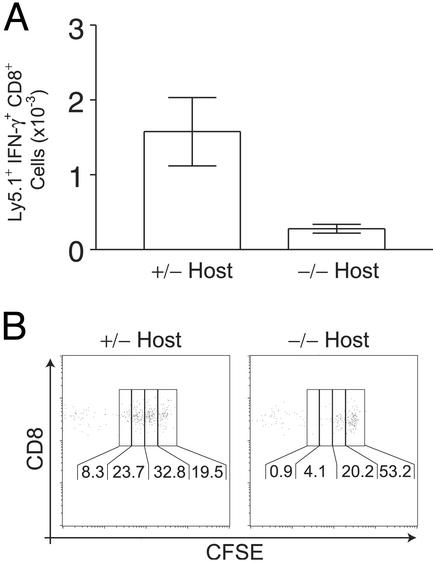
Figure 4.
IL-15Rα−/− mice fail to properly maintain IL-15Ra+/− memory CD8+ T cells. (A) Reduced numbers of IFN-γ+ IL-15Rα+/− memory CD8+ T cells are recovered from IL-15Rα−/− mice 110 days after secondary transfer. The mean total number of Ly5.1+ IFN-γ+ cells recovered from both spleen and lymph nodes are shown ± SEM. Data represent three mice. (B) IL-15Rα+/− memory CD8+ T cells undergo reduced proliferation 55 days after secondary transfer into IL-15Rα−/− hosts. FACS plots are representative of at least two mice. Plots are gated on Ly5.1+ CD8+ T cells and the percentage in each generation is indicated.
Memory CD8+ T cells continuously proliferate in vivo in the absence of cognate antigens, and this proliferation is thought to be important for the long-term maintenance of this pool of cells. To determine whether IL-15Rα supports memory CD8+ T cells by supporting their proliferation, memory IL-15Rα+/− OT-1+ CD8+ T cells from immunized normal mice were labeled with CFSE before transfer into either IL-15Rα+/− or IL-15Rα−/− Ly5.2+ mice. Analyses of lymph node cells from these mice 50 days after transfer revealed that significantly more IL-15Rα+/− memory OT-1+ CD8+ T cells proliferated in IL-15Rα+/− than in IL-15Rα−/− mice (Fig. 4B). Thus, IL-15Rα signals on cells other than the memory cells themselves tonically support memory CD8+ T cell proliferation and the maintenance of the memory CD8+ T cell pool.
T Cell-Independent IL-15Rα Expression on Either Radiation-Sensitive or Radiation-Resistant Cells Supports Memory OT-1+ CD8+ T Cells.
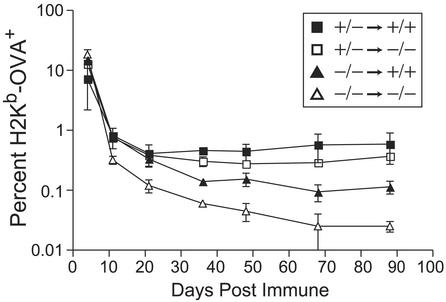
The observation that T cell-independent IL-15Rα signals are critical for memory CD8+ T cells contrasts with prior notions that high-affinity IL-15 receptors on these cells support their proliferation (7, 12–14). As multiple cell types express IL-15Rα, there are many IL-15Rα-dependent cells that could support memory CD8+ T cells. Accordingly, we generated radiation bone marrow chimera to test whether IL-15Rα signals on radiation-resistant cells, radiation-sensitive cells, or both, might be critical for supporting memory OT-1+ CD8+ T cells. Bone marrow cells from either IL-15Rα+/− or IL-15Rα−/− mice were used to reconstitute lethally irradiated congenic IL-15Rα+/− or IL-15Rα−/− Ly 5.2+ mice. Four months after reconstitution, OT-1+ CD8+ T cells were adoptively transferred into these bone marrow chimera, and responses of these mice to poly(I⋅C) plus OVA were analyzed. Irradiated IL-15Rα−/− mice reconstituted with IL-15Rα+/− bone marrow (WT BM→KO chimera) were able to generate and maintain similar numbers of OT-1+ CD8+ memory T cells as irradiated IL-15Rα+/− mice reconstituted with IL-15Rα+/− bone marrow (WT BM→WT chimera; Fig. 5). This ability of bone marrow-derived IL-15Rα+/− hematopoietic cells to rescue the capacity of IL-15Rα−/− mice to generate and support CD8+ memory T cells suggests that radiation-sensitive hematopoietic cells can support CD8+ memory T cells. In addition, IL-15Rα+/− mice reconstituted with IL-15Rα−/− bone marrow cells (KO BM→WT chimera) were also able to generate and maintain significant numbers of OT-1+ CD8+ memory T cells up to 88 days postimmunization, albeit at somewhat lower levels (Fig. 5). The greater capacity of WT BM→WT and WT BM→KO chimera to support OT-1+ CD8+ memory T cells when compared with KO BM→WT chimera is at least partly because of differential support of memory cell proliferation, because in vivo BrdUrd labeling studies revealed both reduced numbers and reduced percentages of memory T cells in the KO BM→WT chimera (see Fig. 9, which is published as supporting information on the PNAS web site). Thus, IL-15Rα expression on radiation-sensitive cells, and, to a lesser extent, radiation-resistant cells, can support the proliferative maintenance of antigen-experienced memory CD8+ T cells.
Figure 5.
IL-15Rα expression on both radiation-sensitive and radiation-resistant cells maintains memory CD8+ T cells. IL-15Rα+/+ (filled squares or triangles) or IL-15Rα−/− (open squares or triangles) mice were lethally irradiated and reconstituted with either IL-15Rα+/− (squares) or IL-15Rα−/− (triangles) bone marrow. At least 4 months after irradiation, mice received OT-1+ RAG−/− CD8+ T cells, and were immunized with OVA and poly(I⋅C). Data represent mean ± SEM of PBLs from at least two mice per group, with most reflecting three or more mice.
Plate-Bound IL-15Rα/IL-15 Can Support IL-15Rα−/− OT-1+ CD8+ T Cells.
IL-15 has repeatedly been shown to directly support the survival and proliferation of purified CD8+ T cells (7). In addition, IL-2Rα was reported to present IL-2 in trans to cells bearing low-affinity IL-2Rβ/γc receptors (24). Most recently, IL-15Rα has been reported to present IL-15 in trans to cells bearing the same IL-2Rβ/γc receptors (25). Thus, one way to reconcile these prior observations with our current results demonstrating a critical and non-cell-autonomous role for IL-15Rα in supporting memory CD8+ T cells is to hypothesize that IL-15Rα on the surface of other cells presents IL-15 in trans to IL-2Rβ/γc receptors on the surface of memory CD8+ T cells. If this occurred in vivo, then IL-15Rα expression would be required on cells that were in contact with memory CD8+ T cells, and would be dispensable on memory CD8+ T cells.
To test this hypothesis in a system where the presenting molecule could only be IL-15 (versus other ligands produced by IL-15Rα competent non-T cells), we asked whether plate-bound IL-15Rα/IL-15 complexes could support memory OT-1+ CD8+ T cells. Thus, IL-15Rα−/− or IL-15Rα+/− OT-1+ CD8+ T cells were transferred into congenic Ly5.2+ mice and immunized with poly(I⋅C) plus OVA. Forty days later, lymph node T cells purified from these mice bearing IL-15Rα−/− or IL-15Rα+/− OT-1+ CD8+ memory T cells were incubated on plates coated with rmIL-15Rα/Fc fusion protein and/or IL-15 (plated at 10 or 100 ng/ml). Analyses of cells recovered from these plates 4 days later revealed that wells coated with both rmIL-15Rα/Fc fusion protein and IL-15, but not with rmIL-15Rα/Fc fusion protein or IL-15 alone, supported the survival of these purified T cells (Fig. 6A). This result suggests that IL-15Rα/IL-15 complexes support T cells in trans. Among these surviving T cells, the proportion of CD44Hi memory phenotype CD8+ T cells and memory OT-1+ CD8+ T cells increased in wells containing both rmIL-15Rα/Fc and IL-15. Thus, both memory phenotype and true memory OT-1+ CD8+ T cells were preferentially supported over naïve CD44Lo CD8+ T cells or CD4+ T cells in the same wells, suggesting that IL-2RβHi T cells selectively benefit from trans presentation of IL-15 (Fig. 6 B and C). Furthermore, plate-bound IL-15Rα/IL-15 complexes comparably supported both IL-15Rα−/− and IL-15Rα+/− OT-1+ CD8+ memory T cells (Fig. 6C). Finally, this support is eliminated by coincubation with anti-IL-2Rβ-specific antibody (Fig. 6). This result is consistent with our in vivo findings demonstrating that IL-15Rα expression on CD8+ memory T cells is dispensable for their survival and proliferation, and suggests that IL-15Rα-bound IL-15 supports CD8+ memory T cells by binding to IL-2Rβ/γc dimeric receptor complexes on these cells. Thus, IL-15Rα can present IL-15 in trans to IL-15Rα−/− CD8+ memory T cells.
Figure 6.
Trans presentation of IL-15 by IL-15Rα can support memory CD8+ T cell survival in vitro. (A) IL-15 presented in trans by IL-15Rα supports T cell viability in vitro. The number of viable cells after 4 days is reported as a percentage of total collected events. (B) IL-15 presented in trans by IL-15Rα selectively supports memory phenotype CD8+ T cells. The percentage of total viable cells that are CD44Hi CD8+ T cells after 4 days of culture is indicated. (C) IL-15Rα-bound IL-15 comparably supports both IL-15Rα+/− and IL-15Rα−/− memory OT-1+ RAG−/− CD8+ T cells in vitro. T cells were purified from WT mice that had received either IL-15Rα+/− or IL-15Rα−/− OT-1+ RAG−/− CD8+ T cells and been immunized with poly(I⋅C) and OVA 40 days before isolation. The percentage of total viable cells that are memory OT-1+ CD8+ T cells after 4 days in culture is indicated. The bars labeled Input reflect the percentage of such cells before culture. This experiment was performed three times with similar results.
Discussion
In this study, we have used a T cell adoptive transfer system to normalize the precursor frequencies of antigen-specific CD8+ T cells and dissect the roles of the high-affinity IL-15Rα chain in supporting CD8+ T cell responses. The essentially complete loss of memory OT-1+ CD8+ T cells in our poly(I⋅C)- plus OVA-immunized IL-15Rα−/− mice contrasts with the nonredundant, but partial, role of IL-15 and IL-15Rα in supporting virally induced memory CD8+ T cells (11–13). As a T cell adjuvant that mimics viral RNA, poly(I⋅C) might be expected to stimulate innate immune responses similar to those initiated by live viruses. However, poly(I⋅C) induces a lower number of antigen-specific CD8+ T cells than do live viruses, so viruses may either induce greater numbers of memory CD8+ T cells that remain detectable for longer periods of time or may induce additional subsets of memory CD8+ T cells. When considered with recent studies (11–13) suggesting that IL-7 and IL-15 can both contribute to the support of memory CD8+ T cells induced by live viruses, the differences between these models may provide important clues regarding the roles of these cytokines in memory T cell generation or maintenance.
Our studies have also distinguished the roles of IL-15Rα expression on T cells versus other cells. Whereas recent studies showed that normal IL-2RβHi memory phenotype CD8+ T cells depend on IL-15 for survival (26) and that IL-15 and IL-15Rα are important for virally induced antigen-specific memory CD8+ T cells, these studies either suggested that IL-15 supported memory CD8+ T cells by binding to IL-15Rα on these cells (7, 12, 13), or they did not address this issue (11). IL-15Rα expression is induced on CD8+ T cells by activation, suggesting it may play an important role in supporting these cells (17). However, the requirement for IL-15Rα expression on memory CD8+ T cells had not been formally addressed. Thus, our dual findings that IL-15Rα expression on CD8+ T cells has no effect on the generation or maintenance of memory CD8+ T cells, whereas IL-15Rα expression on other cells is critical for these cells, surprisingly demonstrate that IL-15Rα supports memory CD8+ T cells entirely through non-cell-autonomous mechanisms.
A wide range of cell types express IL-15Rα, including lymphocytes, myeloid cells, and nonhematopoietic cells. Our experiments with WT BM→KO radiation chimera indicate that IL-15Rα-competent hematopoietic cells can support memory CD8+ T cells. In addition, our KO BM→WT radiation chimera suggest that radiation-resistant cells can also support memory CD8+ T cells. These experiments leave open several possible mechanisms by which IL-15Rα supports memory CD8+ T cells. As IL-7 supports memory CD8+ T cells by directly binding to these cells, and as stromal cells can elaborate IL-7, one possible mechanism by which IL-15Rα might indirectly support memory CD8+ T cells is by stimulating secretion of IL-7. However, RNase protection analyses indicate that IL-7 mRNA levels are comparable in spleens from IL-15Rα+/− and IL-15Rα−/− mice (17). Moreover, recent studies demonstrate that IL-15 and IL-7 deficiency contribute independently to the maintenance of both memory phenotype and memory CD8+ T cells (12, 14). Thus, it is likely that IL-15Rα supports memory CD8+ T cells through IL-7-independent mechanisms.
A second possible mechanism is that IL-15Rα expression on other cells stimulates the further secretion of IL-15. In this scenario, reduced levels of soluble IL-15 might be present in IL-15Rα−/− mice and thus lead to reduced stimulation of IL-15 receptors on T cells. However, IL-15 does not induce IL-15 mRNA (at least not in macrophages; ref. 7), total IL-15 mRNA levels are similar in spleens from IL-15Rα+/− and IL-15Rα−/− mice (17), and RT-PCR analyses of various tissues from IL-15Rα+/− and IL-15Rα−/− mice failed to reveal any reduction of the spliced form of IL-15 mRNA, which encodes secreted IL-15 protein (data not shown) (27).
A third possibility is that IL-15Rα on the surface of other cells presents IL-15 in trans to memory CD8+ T cells (25). Our data support this intriguing mechanism by demonstrating that plate-bound IL-15/IL-15Rα complexes, but not plate-bound IL-15, can support IL-15Rα−/− OT-1+ CD8+ T cells. This finding is entirely consistent with recent experiments demonstrating that IL-15Rα on the surface of monocytes can recycle membrane-bound IL-15 through endosomes and can present IL-15 in trans to IL-15Rβ/γc-receptor-bearing PT-18 cells in vitro (25). Our data suggest that trans presentation of IL-15 not only is physiologically relevant but may in fact be the dominant mechanism by which IL-15Rα supports memory CD8+ T cells in vivo. Thus, memory CD8+ T cells may require periodic stimulation of their IL-15Rβ/γc-receptor complexes by surface-bound IL-15Rα/IL-15 on neighboring cells. We have not excluded the possibility that IL-15Rα-initiated signals on these neighboring cells may also induce the expression of other molecules that support memory CD8+ T cells. However, our experiments with purified recombinant IL-15Rα-bound IL-15 demonstrates that the supportive interaction of this complex with purified T cells can occur in the absence of other accessory cells. Thus, the most straightforward interpretation of these data is that the proliferative maintenance of memory CD8+ T cells can be sustained by periodic exposure of these cells to IL-15Rα-expressing cells presenting IL-15 in trans.
In summary, our experiments have demonstrated that IL-15Rα expression on CD8+ T cells is dispensable for the generation and maintenance of memory CD8+ T cells. This surprising finding runs counter to the widely held notion that soluble IL-15 supports memory CD8+ T cells by binding to high-affinity IL-15Rα receptors on these cells. By contrast, our results show that IL-15Rα expression on other cells is critical for supporting memory CD8+ T cells. This non-cell-autonomous role for IL-15Rα expression may be related to the ability of IL-15Rα to present IL-15 in trans to IL-15Rβ/γc low-affinity receptors. These findings provide insights into how IL-15Rα supports memory T cells in vivo and suggest that IL-15-based vaccine strategies might be better targeted at cells other than T cells.
Supplementary Material
Acknowledgments
We thank T. A. Waldmann for generously communicating data before publication. This work was supported by National Institutes of Health Grant AI45860 and Digestive Diseases Research Center Grant DK42086, Training Grants GM07281 (to P.R.B.) and AI07090 (to R.K.), the Crohn's and Colitis Foundation of America (to D.L.B.), the Mr. and Mrs. Arthur Edelstein FACS Facility, and the Martin Boyer Laboratories.
Abbreviations
- poly(I⋅C)
poly(inosinic acid)⋅poly(cytidylic acid)
- OVA
ovalbumin
- PBL
peripheral blood lymphocyte
- IL-15R
IL-15 receptor
- CFSE
carboxyfluorescein diacetate-succinimidyl ester
- rmIL
recombinant murine IL
- WT BM→KO chimera
irradiated (KO) mice reconstituted with wild-type bone marrow
- RAG
recombinase-activating gene
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 2.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 4.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 8.Fehniger T A, Suzuki K, Ponnappan A, VanDeusen J B, Cooper M A, Florea S M, Freud A G, Robinson M L, Durbin J, Caligiuri M A. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 11.Becker T C, Wherry E J, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldrath A W, Sivakumar P V, Glaccum M, Kennedy M K, Bevan M J, Benoist C, Mathis D, Butz E A. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns K S, Williams K, Ma A, Zheng X X, Lefrancois L. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 14.Tan J T, Ernst B, Kieper W C, LeRoy E, Sprent J, Surh C D. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 16.Willerford D M, Chen J, Ferry J A, Davidson L, Ma A, Alt F W. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 17.Lodolce J P, Burkett P R, Boone D L, Chien M, Ma A. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boone D L, Dassopoulos T, Lodolce J P, Chai S, Chien M, Ma A. Inflamm Bowel Dis. 2002;8:35–42. doi: 10.1097/00054725-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 20.Kim S K, Reed D S, Olson S, Schnell M J, Rose J K, Morton P A, Lefrancois L. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masopust D, Vezys V, Marzo A L, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 22.Reinhardt R L, Khoruts A, Merica R, Zell T, Jenkins M K. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 23.Manjunath N, Shankar P, Wan J, Weninger W, Crowley M A, Hieshima K, Springer T A, Fan X, Shen H, Lieberman J, von Andrian U H. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eicher D M, Waldmann T A. J Immunol. 1998;161:5430–5437. [PubMed] [Google Scholar]
- 25.Dubois S, Mariner J, Waldmann T A, Tagaya Y. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 26.Judge A D, Zhang X, Fujii H, Surh C D, Sprent J. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattei F, Schiavoni G, Belardelli F, Tough D F. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.