Abstract
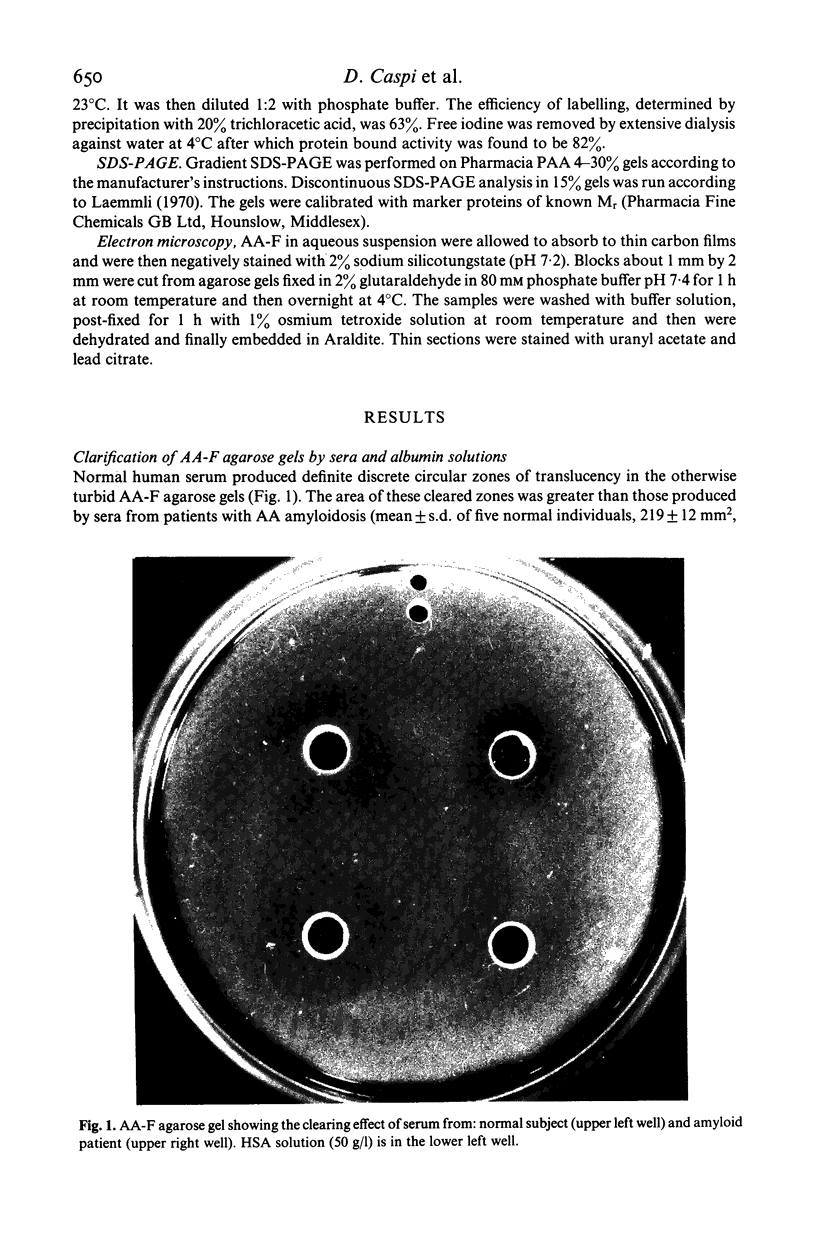
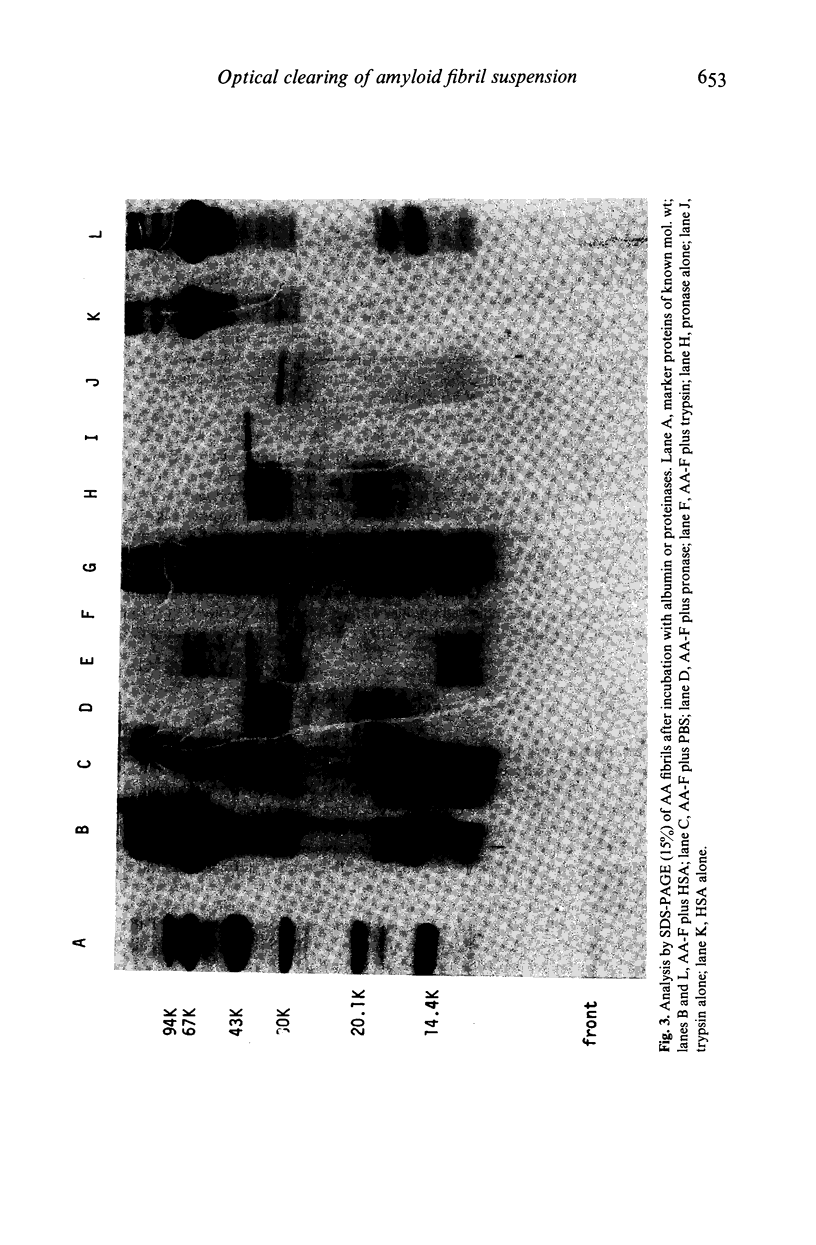
Clearing of turbid amyloid A fibril containing agarose gels by human serum has been ascribed to 'amyloid degrading activity'. We report here that this optical phenomenon is not due to an enzymatic reaction, does not involve proteolysis of the fibril subunits and is not inhibited by sera of patients with AA amyloidosis. The extent of clearing correlates closely with the serum albumin concentration and, as previously reported by others, serum albumin itself causes clearing comparable to that of whole serum. Furthermore addition of albumin solutions to turbid aqueous suspensions of AA amyloid fibrils causes immediate clearing. Serum albumin is known to clarify turbid non-amyloid fibril containing gels and is used commercially to improve the optical properties of radial immunodiffusion plates. We therefore propose that this property of albumin, the mechanism of which is not yet understood, underlies the so called 'amyloid degrading activity' of human serum and the latter is not therefore likely to be of in vivo biological or clinical significance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz M. L., Caspi D., Glatthaar B. E., Moser U., Pepys M. B. The failure of ascorbic acid therapy to alter the induction or remission of murine amyloidosis. Clin Exp Immunol. 1984 Sep;57(3):657–662. [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Cohen A. S. Serum amyloid A protein in amyloidosis, rheumatic, and enoplastic diseases. Arthritis Rheum. 1979 Jan;22(1):36–42. doi: 10.1002/art.1780220106. [DOI] [PubMed] [Google Scholar]
- De Beer F. C., Mallya R. K., Fagan E. A., Lanham J. G., Hughes G. R., Pepys M. B. Serum amyloid-A protein concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet. 1982 Jul 31;2(8292):231–234. doi: 10.1016/s0140-6736(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Kedar I., Sohar E., Ravid M. Degradation of amyloid by a serum component and inhibition of degradation. J Lab Clin Med. 1982 May;99(5):693–700. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Mechanism of reduced amyloid-A-degrading activity in serum of patients with secondary amyloidosis. Lancet. 1982 Jul 31;2(8292):234–237. doi: 10.1016/s0140-6736(82)90322-1. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salacinski P. R., McLean C., Sykes J. E., Clement-Jones V. V., Lowry P. J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal Biochem. 1981 Oct;117(1):136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- Teppo A. M., Maury C. P., Wegelius O. Characteristics of the amyloid A fibril-degrading activity of human serum. Scand J Immunol. 1982 Oct;16(4):309–314. doi: 10.1111/j.1365-3083.1982.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Wegelius O., Teppo A. M., Maury C. P. Reduced amyloid-A-degrading activity in serum in amyloidosis associated with rheumatoid arthritis. Br Med J (Clin Res Ed) 1982 Feb 27;284(6316):617–619. doi: 10.1136/bmj.284.6316.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. R., Calkins E., Humphrey R. L. Potassium permanganate reaction in amyloidosis. A histologic method to assist in differentiating forms of this disease. Lab Invest. 1977 Mar;36(3):274–281. [PubMed] [Google Scholar]




