Abstract
Recessive Stargardt's macular degeneration is an inherited blinding disease of children caused by mutations in the ABCR gene. The primary pathologic defect in Stargardt's disease is accumulation of toxic lipofuscin pigments such as N-retinylidene-N-retinylethanolamine (A2E) in cells of the retinal pigment epithelium. This accumulation appears to be responsible for the photoreceptor death and severe visual loss in Stargardt's patients. Here, we tested a therapeutic strategy to inhibit lipofuscin accumulation in a mouse model of recessive Stargardt's disease. Isotretinoin (Accutane) has been shown to slow the synthesis of 11-cis-retinaldehyde and regeneration of rhodopsin by inhibiting 11-cis-retinol dehydrogenase in the visual cycle. Light activation of rhodopsin results in its release of all-trans-retinaldehyde, which constitutes the first reactant in A2E biosynthesis. Accordingly, we tested the effects of isotretinoin on lipofuscin accumulation in abcr−/− knockout mice. Isotretinoin blocked the formation of A2E biochemically and the accumulation of lipofuscin pigments by electron microscopy. We observed no significant visual loss in treated abcr−/− mice by electroretinography. Isotretinoin also blocked the slower, age-dependent accumulation of lipofuscin in wild-type mice. These results corroborate the proposed mechanism of A2E biogenesis. Further, they suggest that treatment with isotretinoin may inhibit lipofuscin accumulation and thus delay the onset of visual loss in Stargardt's patients. Finally, the results suggest that isotretinoin may be an effective treatment for other forms of retinal or macular degeneration associated with lipofuscin accumulation.
Fine central vision in humans is mediated by an area of the retina called the macula, which contains a high density of rod and cone photoreceptors. This region is vulnerable to degeneration in a group of central blinding diseases called the macular degenerations. Age-related macular degeneration, for example, is a common disease of complex etiology that causes reduced visual acuity and legal blindness in the elderly (1, 2). Recessive Stargardt's disease is an inherited form of macular degeneration with an onset of central visual loss during childhood (3) and an estimated prevalence of ≈1/10,000 (4). Pathologically, Stargardt's disease is associated with accumulation of fluorescent lipofuscin pigments in cells of the retinal pigment epithelium (RPE) (5, 6). The RPE is a layer of cells adjacent to the retina that plays a critical role in photoreceptor survival (7). No effective treatments exist for age-related or Stargardt's macular degeneration.
The gene affected in recessive Stargardt's disease, ABCA4 or ABCR, encodes a transporter protein in the rims of rod and cone outer-segment discs (8–10). To study the function of the ABCR protein and the molecular etiology of Stargardt's disease, we previously generated mice with a knockout mutation in the abcr gene. These animals have a complex ocular phenotype that includes elevated all-trans-retinaldehyde (atRAL) after light exposure and accumulation of lipofuscin pigments in cells of the RPE (11–13). These animals also manifest very slow photoreceptor degeneration (13). Based on biochemical analysis of abcr−/− mice (11–13) and the results of in vitro studies (14, 15), ABCR appears to function as an outwardly directed flippase for N-retinylidene-phosphatidylethanolamine (N-ret-PE), the Schiff-base conjugate of phosphatidylethanolamine and atRAL. Accordingly, ABCR may play a role in the visual cycle for the regeneration of rhodopsin (Fig. 1A) by accelerating removal of atRAL from outer-segment discs (11).
Figure 1.
Retinoid pathways in the retina and RPE. (A) Visual cycle mediating rhodopsin regeneration. Absorption of a photon (hv) by a rhodopsin molecule in a rod outer-segment disk induces photoisomerization of the 11cRAL chromophore, yielding activated metarhodopsin II. After several seconds, metarhodopsin II decays to yield apo-rhodopsin and free atRAL. Elimination of atRAL from the interior of outer-segment discs is facilitated by the ABCR transporter (11). The atRAL subsequently is reduced to atROL (vitamin A) by atROL dehydrogenase (atRDH). The atROL is released from the outer segment and taken up by an RPE cell, where it is esterified by lecithin retinol acyl transferase (LRAT) to form an all-trans-retinyl ester (atRE). Chemical isomerization is effected by isomerohydrolase (IMH), which uses atRE as a substrate. The resulting 11cROL is oxidized by 11cRDH to form 11cRAL chromophore. 11cRDH is inhibited by isotretinoin with a Ki of ≈0.1 μM (26, 27). The Ki for inhibition of atRDH by isotretinoin is at least two logs higher (N.L.M., R.A.R., and G.H.T., unpublished observations). 11cROL also may serve as a substrate for LRAT to form 11cRE. The final step is recombination of 11cRAL with apo-rhodopsin in the outer segment to form a new molecule of light-sensitive rhodopsin. (B) Synthesis of A2E. After light exposure, newly released atRAL condenses reversibly with phosphatidylethanolamine to form N-ret-PE (step 1). Rarely, a second molecule of atRAL will condense with N-ret-PE to form A2PE-H2 (step 2). The wavelength of maximal absorption (λmax) for A2PE-H2 is 500 nm. Within the acidic and oxidizing environment of RPE phagolysosomes, A2PE-H2 is oxidized to N-retinylidene-N-retinylphosphatidylethanolamine (A2PE; λmax = 435 nm; step 3). Finally, hydrolysis of the phosphate ester yields A2E (λmax = 435 nm) and phosphatidic acid (step 4) (12).
The major fluorescent component of lipofuscin is the bis-retinoid pyridinium salt N-retinylidene-N-retinylethanolamine (A2E) (16, 17). Significant accumulation of A2E is seen in RPE cells from abcr−/− mice (11, 12) and patients with Stargardt's disease (5, 6, 12). A2E forms by sequential condensation of atRAL with phosphatidylethanolamine (Fig. 1B) (12, 18), both of which are elevated in abcr−/− retinas (11, 12). A2E sensitizes RPE cells to light-induced apoptosis (19–21) and has an inhibitory effect on phospholipid turnover in RPE phagolysosomes (22). Central blindness in Stargardt's patients results from photoreceptor degeneration caused by A2E-mediated toxicity to the RPE (11, 21). Accordingly, a reasonable strategy to slow the progression of visual loss in Stargardt's patients is to inhibit formation of A2E. Isotretinoin (13-cis-retinoic acid or Accutane) is a drug in common use for the treatment of acne (23). A side effect of treatment with isotretinoin is reduced night vision (24, 25) because of its inhibitory effect on 11-cis-retinol dehydrogenase (11cRDH) in RPE cells (26, 27) (Fig. 1A). Treatment of rodents with isotretinoin was shown to delay rhodopsin regeneration and slow recovery of rod sensitivity after light exposure (28). Importantly, isotretinoin did not cause photoreceptor degeneration and actually protected photoreceptors from light-induced damage (28). These observations suggest that isotretinoin may safely inhibit formation of A2E in Stargardt's patients by reducing levels of its molecular precursors. In the current study, we investigated this potential treatment strategy in abcr−/− mice.
Materials and Methods
Mice.
For all studies except the experiment represented in Fig. 6, mice were maintained under 12-hr cyclic light with an average illuminance of 10 lux inside the cages. For the study represented in Fig. 6, mice were maintained under 12-hr cyclic light with an average cage illuminance of 30 lux. Wild-type (strain C57BL/6) and abcr−/− (B6 × 129 hybrid) mice were administered isotretinoin (Sigma) at 20 or 40 mg per kg of body weight in 25 μl of DMSO by i.p. injection. Control mice received 25-μl injections of DMSO alone. To determine dihydro-N-retinylidene-N-retinylphosphatidylethanolamine (A2PE-H2) and A2E in RPE, abcr−/− mice were implanted s.c. for 1 month with a modified (29) Alzet model 2004 osmotic pump (Palo Alto, CA) containing 200 μl of isotretinoin (160 mg/ml) in DMSO (delivery rate = 0.25 μl/hr). To measure acute formation of N-ret-PE, 1.0 μCi [3H]atROL (all-trans-retinol; 2.0 pmol per μCi) in 1.0 μl of DMSO was injected into the vitreous cavities of mice by using a 33-gauge needle under direct visualization. All mice were anesthetized by i.p. injection of ketamine (200 mg/kg) plus xylazine (10 mg/kg) before electroretinography (ERG) analysis, intravitreal injections, and death by cervical dislocation.
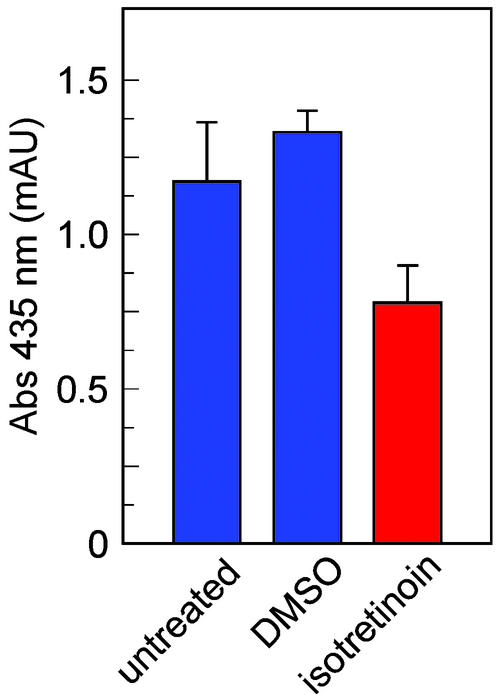
Figure 6.
Levels of A2E in 4-month-old wild-type mice after 2 months of treatment with isotretinoin. A2E levels expressed as peak-area mAU (mAU at λ = 435 nm) are shown in RPE from untreated mice (blue bar, n = 4 mice), from mice that received daily injections of DMSO vehicle (blue bar, n = 3), and from mice that received daily injections of isotretinoin at 20 mg/kg (red bar, n = 4). Error bars show SD.
ERG Analysis.
Dark-adapted mice received a single i.p. injection of isotretinoin at 40 mg/kg. One hour later, mice were anesthetized with ketamine/xylazine. ERGs were recorded from the corneal surface of one eye after pupil dilation (1% atropine sulfate), as described (30). Responses were amplified (Grass CP511 AC amplifier; Grass Instruments, Quincy, MA; ×10,000; 3 dB down at 2 and 10,000 Hz) and digitized by using a National Instruments PCI-1200 I/O board (Austin, TX). Mice were kept on a heated pad at 38°C. The recovery of dark-adapted function in treated and untreated mice was measured after a 25-s exposure to 1,000-lux white light that bleached ≈40% of rhodopsin. The time course of rod recovery in the dark was monitored by measuring rod ERG amplitudes normalized to the prebleach amplitude. In some experiments, a dim achromatic probe flash (−0.91 log scotopic td-s) that produced only a definable b-wave (no a-wave) was used. In other experiments, a bright probe flash (Kodak Wratten 47B; 3.11 log scotopic td-s) that produced a saturated (a-wave) photoresponse was used. The leading edges of the a-waves (before and after photobleach) were fitted with a computational model to provide estimates of photoreceptor activity, as described (31, 32). The estimate of RmP3 (Fig. 2 C and D) corresponds to the maximum saturated photovoltage derived from the fit of this model. Mice treated with isotretinoin also were tested 2 days before treatment, allowing direct measurement of the treatment effect in the same animal.
Figure 2.
Analysis of visual function by ERG in wild-type and abcr−/− mice after treatment with isotretinoin. (A) b-wave amplitudes elicited in wild-type mice with a dim probe flash (−0.91 log scot td-s) at the indicated times after a 40% photobleach divided by the dark-adapted b-wave amplitude. The mice received a single injection of isotretinoin (40 mg/kg) 1 hr before the photobleach (red circles, n = 7) or received no treatment (blue circles, n = 4). (B) Same protocol as in A except mice were abcr−/− genotype (red circles, n = 4; blue circles, n = 5). (C) RmP3 amplitudes (derived from the leading edge of the a-wave) elicited in wild-type mice with a bright probe flash (3.11 log scot td-s) at the indicated times after a 40% photobleach divided by the dark-adapted RmP3 amplitude. Treatment protocol was similar to A (red circles, n = 7; blue circles, n = 7). (D) Same protocol as in C except mice were abcr−/− genotype (red circles, n = 9; blue circles, n = 9). Error bars show SD.
Tissue Preparation and Extraction.
After death, eyes were removed and hemisected. For analysis of 11-cis-retinyl ester (11cRE), A2PE-H2, and A2E, retinas, RPE, or eyecups containing retinas plus RPE were dissected and homogenized in PBS, pH 7.2. For 11cRE, 1.0 ml of ethanol was added and the samples were extracted twice in 3.0 ml of hexane. For A2PE-H2 and A2E, 4.0 ml of chloroform/methanol (2:1, vol/vol) was added and the samples were homogenized. All samples were extracted with the addition of 4.0 ml of chloroform and 3.0 ml of distilled water and centrifuged at 1,000 × g for 10 min. For analysis of 11-cis-retinaldehyde (11cRAL) and N-ret-PE, tissues were homogenized in 0.1 M potassium phosphate buffer (pH 7.0) containing 6.0 M formaldehyde (33). Two milliliters of methylene chloride was added to homogenates followed by incubation at 30°C for 10 min. Samples of 11cRAL were extracted twice in 3.0 ml of hexane and prepared for HPLC as described above. One milliliter of methanol was added to the N-ret-PE, and the samples were extracted with addition of 4.0 ml of chloroform and 3.0 ml distilled water and centrifuged at 1,000 × g for 10 min. The organic phases were removed and the samples were dried under a stream of argon. The residues were redissolved in 100 μl of hexane and analyzed by HPLC. All tissue manipulations were done on ice under dim red light (Kodak Wratten 1A).
HPLC.
A2E and A2PE-H2 samples were analyzed by normal-phase HPLC on a silica column (Microsorb 5 μm Si, 250 × 4.6 mm) (Sigma) with the mobile phase: hexane/2-propanol/ethanol/25 mM potassium phosphate/acetic acid (485:376:100:50:0.275 vol/vol) in an Agilent (Wilmington, DE) model 1100 liquid chromatograph with photodiode-array detector. Column and solvent temperatures were maintained at 30°C. Chromatographic conditions and quantitation of A2E were as described (11, 12). Samples containing 11cRAL, 11cRE, and [3H]N-ret-PE were analyzed by normal-phase HPLC by using gradient elution (0.2–10% dioxane in hexane at 2 ml/min) on a similar column. The chromatograph was equipped with an online flow-scintillation analyzer (Packard 505TR) configured to monitor 3H-labeled compounds in the energy range of 0–15 keV as described (34).
Electron Microscopy.
One group of three 2-month-old abcr−/− mice were treated with daily i.p. injections of isotretinoin at 20 mg/kg for 2 months. A similar control group received no treatment. All mice were anesthetized with ketamine/xylazine and perfused through the heart with 2.0% paraformaldehyde and 1.0% glutaraldehyde in PBS. Fixed eyes were removed and sectioned along the ora serrata, and eyecups were immersed in 2.0% glutaraldehyde and 2.0% paraformaldehyde in 100 mM cacodylate buffer (pH 7.4) overnight at 4°C. Fixed eyecups were dehydrated through an ethanol series to 100% and embedded in Poly/Bed 812 epoxy resin (Polysciences, Warrington, PA). Ultrathin sections (60 nm) from the central region of the eye were cut with a Leica Ultracut UCT microtome (Deerfield, IL), stained with 5.0% uranyl acetate and lead citrate, and examined with a JEOL 1200EX transmission electron microscope.
Results
Isotretinoin Causes Delayed Dark Adaptation in Wild-Type and abcr−/− Mice.
All vertebrates experience a period of reduced visual sensitivity after light exposure, called dark adaptation. As described (28), wild-type mice show delayed dark adaptation after a single dose of isotretinoin. We tested the effects of isotretinoin in 4-month-old wild-type and abcr−/− mice by ERG, which records the electrical response of the retina to a light flash. Mice were dark-adapted overnight and administered a single i.p. dose of isotretinoin at 40 mg/kg. One hour after injection, the mice were exposed to light that bleached ≈40% of rhodopsin. We analyzed mice by ERG immediately before and at different times after the photobleach. In one set of experiments, we used dim probe flashes to elicit the ERGs. Both wild-type and abcr−/− mice dark-adapted more slowly after isotretinoin administration (Fig. 2 A and B). Untreated abcr−/− mice dark-adapted more slowly than wild-type mice, as described (11). In another set of experiments, we used bright probe flashes to elicit the ERGs. Here, we observed much smaller differences in dark adaptation between treated and untreated mice of both genotypes (Fig. 2 C and D). Finally, we recorded ERGs without prior photobleach from 4-month-old, dark-adapted wild-type and abcr−/− mice that were either untreated, treated with a single i.p. injection of isotretinoin at 40 mg/kg 1 hr before analysis, or treated for 2 months with daily i.p. injections of isotretinoin at 20 mg/kg. The dark-adapted ERGs were indistinguishable between treated and untreated mice of both genotypes (data not shown), indicating that isotretinoin did not cause abnormalities in dark-adapted visual function.
Effects of Isotretinoin on the Levels of Visual Retinoids.
We examined the levels of 11cRAL and 11cRE by HPLC in eyecups (retina + RPE) from 4-month-old light-adapted wild-type mice 1 hr after a single i.p. injection of isotretinoin at different doses. As expected, we observed a dose-dependent reduction in 11cRAL (Fig. 3A). 11cRE was undetectable in eyecups from mice that received no isotretinoin but was present in treated mice at levels that increased with the dose (Fig. 3B). No significant differences in total retinoid levels were observed between treated and untreated mice. We also looked at formation of N-ret-PE in 5-month-old abcr−/− mice treated with isotretinoin for 10 days by i.p. injection at 20 mg/kg per day. Three days before sacrifice, we injected a trace amount (2.0 pmol) of [3H]atROL into the vitreous cavity of each eye. After 3 days under cyclic light, we collected eyecups from the injected eyes and assayed for [3H]N-ret-PE by HPLC with an online radiometric detector. Compared with untreated abcr−/− controls, formation of [3H]N-ret-PE was reduced by 40% in the treated eyecups (Fig. 3C).
Figure 3.
Effects of isotretinoin on the content of visual retinoids in wild-type and abcr−/− eyes. (A) Levels of 11cRAL in light-adapted, wild-type mice that received either no isotretinoin (blue bar, n = 3 mice) or a single injection 1 hr before tissue collection of isotretinoin at 2.0 (red bar, n = 2), 20 (red bar, n = 3), or 200 (red bar, n = 3) mg/kg. (B) Levels of 11cRE in light-adapted wild-type mice that received either no isotretinoin or a single injection of isotretinoin at the indicated doses (n = 3) as described in A. Note the absence of 11cRE in untreated eyes. (C) [3H]N-ret-PE dpm expressed as a percentage of the total [3H]dpm in phospholipid extracts from eyecups of abcr−/− mice that either were untreated (blue bar, n = 3) or were treated with isotretinoin at 20 mg/kg per day for 10 days (red bar, n = 4). Error bars show SD.
Sustained Treatment with Isotretinoin Inhibits Accumulation of A2PE-H2 and A2E in abcr−/− RPE.
Reduced formation of N-ret-PE in treated retinas suggests that isotretinoin may inhibit accumulation of A2E. To test this possibility, we administered isotretinoin at 40 mg/kg per day via osmotic pump to 3-month-old abcr−/− mice. After 1 month, we killed the mice and determined the levels of A2PE-H2 and A2E in RPE by HPLC. In untreated abcr−/− mice or mice treated with DMSO vehicle alone, A2PE-H2 increased ≈2.7-fold in the period between 3 and 4 months (Fig. 4A). A2E increased 2.3-fold in DMSO-treated controls during the same period (Fig. 4B). However, the levels of both A2PE-H2 and A2E virtually were unchanged from the levels at 3 months in mice that received isotretinoin (Fig. 4). These data suggest that treatment with isotretinoin suppresses A2PE-H2 and A2E accumulation.
Figure 4.
Levels of A2PE-H2 and A2E in abcr−/− RPE after 1 month of treatment with isotretinoin. (A) Levels of A2PE-H2 in RPE from 3-month-old abcr−/− mice before treatment (green bar, n = 3 mice), 4-month-old untreated (blue bar, n = 3) or DMSO-treated (blue bar, n = 3) control mice, and 4-month-old mice treated with 40 mg/kg per day isotretinoin (red bar, n = 3). Values are expressed as peak-height milliabsorption units (mAU) at wavelength (λ) = 500 nm. (B) Levels of A2E in RPE from 3-month-old abcr−/− mice before treatment (green bar, n = 3), 4-month-old DMSO-treated control mice (blue bar, n = 3), and 4-month-old mice treated with 40 mg/kg per day isotretinoin (red bar, n = 3). Values are expressed in mAU at λ = 435 nm. Error bars show SD.
Treatment with Isotretinoin Inhibits Formation of Lipofuscin in abcr−/− RPE.
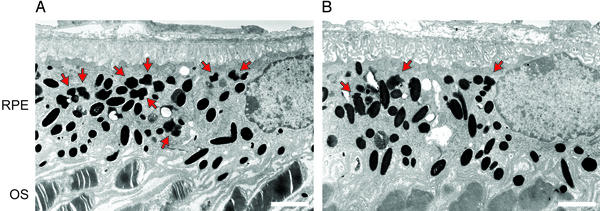
Next, we looked for an ultrastructural correlate of the reduced A2E. We treated 2-month-old abcr−/− mice with isotretinoin at 20 mg/kg per day for 2 months. At the end of the treatment period, we examined the eyes by electron microscopy. Representative electron micrographs of the central retina and RPE from untreated (Fig. 5A) and treated (Fig. 5B) 4-month-old abcr−/− mice are shown. Oval melanosomes were present at approximately equal density in RPE from both mice. However, the irregularly shaped lipofuscin granules were more abundant in the RPE from untreated mice. These data show that isotretinoin treatment inhibits accumulation of lipofuscin pigments.
Figure 5.
Electron microscopic analysis of RPE from 4-month-old abcr−/− mice. (A) RPE and outer segments (OS) from an untreated mouse. (B) RPE and OS from a mouse treated with isotretinoin for 2 months. Red arrows indicate the irregularly shaped lipofuscin pigment granules, which are distinct from the larger oval melanosomes. (Bars, 2.0 μm.) Note the increased number of lipofuscin granules in RPE from the untreated mouse.
Accumulation of A2E in Wild-Type RPE also Is Inhibited by Isotretinoin.
A2E accumulates at slower rates in RPE from wild-type mice and normal humans (12). To test for a possible effect of isotretinoin on this slow, age-dependent accumulation of A2E, we treated 2-month-old, wild-type (C57BL/6) mice with daily i.p. injections of isotretinoin at 20 mg/kg for 2 months. A2E was reduced ≈40% in these 4-month-old mice compared with the levels in littermate controls who received either no treatment or injections of DMSO vehicle (Fig. 6). The A2E-absorption data in Fig. 6 represent a sum of peak areas from the different A2E isoforms. Because of the dissimilar light-exposure histories and A2E-quantitation methods, A2E levels in Figs. 4 and 6 are not directly comparable. Under controlled conditions, A2E levels are ≈10-fold higher in 3-month-old abcr−/− compared with wild-type mice (12). The data in Fig. 6 show that isotretinoin acts independently of the abcr genotype to reduce accumulation of A2E.
Discussion
The primary deficit in abcr−/− mice and patients with Stargardt's disease is functional loss of the ABCR transporter. Impaired clearance of N-ret-PE from outer segments that lack ABCR favors the secondary condensation of N-ret-PE with atRAL to form A2PE-H2 (12) (Fig. 1B). The level of atRAL is ≈1.5-fold higher in abcr−/− compared with wild-type outer segments (11), suggesting that modest reductions in atRAL may be all that are required to inhibit formation of A2PE-H2 and, hence, A2E. In the current study, we sought to inhibit formation of A2E by reducing production of retinaldehyde in outer segments.
Isotretinoin inhibits 11cRDH in RPE cells (26, 27). We analyzed the kinetics of dark adaptation in isotretinoin-treated and untreated mice by ERG with bright and dim probe flashes. Dim flashes provide nonsaturating stimulation of rods, resulting in an ERG response that is dependent on the level of rhodopsin. Bright flashes saturate the rod response and, hence, elicit ERGs less sensitive to rhodopsin levels. Both wild-type and abcr−/− mice showed smaller delays in dark adaptation after isotretinoin administration with bright compared with dim probe flashes (Fig. 2). These results suggest that isotretinoin reduced rhodopsin levels in both wild-type and abcr−/− retinas.
We confirmed the inhibitory effect of isotretinoin on 11cRDH (Fig. 1A) by demonstrating dose-dependent reductions in 11cRAL (Fig. 3A). Because 11-cis-retinol (11cROL) is esterified by LRAT (35, 36), treatment with isotretinoin should result in increased 11cRE, which we observed (Fig. 3B). 11cRE was not detectable in 4-month-old untreated mice, as described (34, 37), presumably because of rapid oxidation of 11cROL by 11cRDH (Fig. 1A). In addition to its effect on 11cRDH, might isotretinoin act by inhibiting transport of 11cRAL between RPE and photoreceptors, because retinoic acid binds interphotoreceptor retinoid-binding protein (38)? This is unlikely in view of the normal rhodopsin regeneration kinetics observed in irbp−/− knockout mice (39). N-ret-PE is nearly 3-fold higher in retinas from abcr−/− compared with wild-type mice because of increased atRAL (11, 12). Lowering atRAL in abcr−/− photoreceptors should result in reduced N-ret-PE. We injected radio-labeled vitamin A into the vitreous cavities of mice that previously received isotretinoin for 1 week and measured levels of labeled N-ret-PE. This design permitted us to consider only N-ret-PE formed during the treatment period. We observed an ≈2-fold reduction in labeled N-ret-PE (Fig. 3). Because N-ret-PE gives rise to A2E (Fig. 1B), this result suggests that long-term treatment with isotretinoin may suppress A2E accumulation. To test this possibility, we measured levels of A2PE-H2 and A2E in 4-month-old abcr−/− RPE after 1 month of treatment. A2E accumulates rapidly in abcr−/− mice between 2 and 4 months of age (12). In the current study, A2PE-H2 increased almost 3-fold and A2E approximately doubled in untreated or DMSO-treated abcr−/− control mice (Fig. 4). However, in animals that received isotretinoin, the levels of A2PE-H2 and A2E at 4 months were virtually unchanged from the starting levels in 3-month-old mice (Fig. 4). Thus, treatment with isotretinoin completely suppressed subsequent accumulation of A2PE-H2 and A2E. Because A2E is the major fluorophore of lipofuscin (16, 17), we also examined the morphological effects of isotretinoin treatment on lipofuscin pigment formation in abcr−/− eyes. We observed significantly fewer pigment granules in RPE from treated animals compared with untreated controls (Fig. 5). We observed no changes in the ultrastructure of outer segments from treated abcr−/− mice. Finally, we looked for an effect of isotretinoin treatment on the much slower accumulation of A2E in wild-type RPE. After 2 months of treatment, A2E in RPE from 4-month-old, C57BL/6 mice was reduced ≈40% compared with untreated or DMSO-treated controls (Fig. 6). These data corroborate the proposed pathway for A2E biogenesis (12, 18) depicted in Fig. 1B. Further, the data establish that treatment with isotretinoin inhibits formation of A2E and lipofuscin in abcr−/− and wild-type mice.
The phenotypes in abcr−/− mice and humans with Stargardt's disease are strikingly similar. Both show delayed dark adaptation, elevated phosphatidylethanolamine in retina, elevated A2PE-H2 and A2E in RPE cells, and accumulation of lipofuscin pigments in RPE (5, 6, 11, 12). Given the physiological, biochemical, morphological, and genetic similarities between abcr−/− mice and Stargardt's patients, a treatment that ameliorates the mouse phenotype should have a similar effect in humans. Previously, we showed that A2E synthesis can be virtually blocked by raising abcr−/− mice in total darkness (12). This observation suggests that Stargardt's patients may slow progression of their disease by limiting light exposure. In the current study, we replicated the effects of light deprivation on A2E and lipofuscin accumulation in abcr−/− mice by inhibiting rhodopsin regeneration with isotretinoin. This treatment was associated with delayed dark adaptation but normal absolute rod-threshold sensitivity after prolonged dark adaptation. Delayed dark adaptation also has been reported in humans taking isotretinoin, although the prevalence of this side effect is difficult to quantitate (24, 25). The dose of isotretinoin in the current study (20–40 mg/kg per day) is much higher than the therapeutic dose used in humans to treat acne (0.5–2.0 mg/kg per day). However, the rate of clearance for isotretinoin is 10- to 20-fold faster in mice than humans (40). We observed significant effects on the levels of 11cRAL and 11cRE after a single injection of isotretinoin at 2.0 mg/kg (Fig. 3 A and B), suggesting that this dose inhibits 11cRDH. Together, these observations suggest that suppression of A2E and lipofuscin accumulation may be achieved in Stargardt's patients with standard doses of isotretinoin. Finally, isotretinoin or other inhibitors of 11cRDH may be effective treatments for retinal or macular degenerations of different etiologies associated with A2E accumulation.
Acknowledgments
We thank Aarti Bagla, Tam Bui, Zahra Kashani, Jung Lee, and Jonathan Moody for outstanding technical support. This work is supported by grants from the National Eye Institute, the Foundation Fighting Blindness, the Ruth and Milton Steinbach Fund, and the Macula Vision Research Foundation. R.A.R. is a postdoctoral fellow on the Jules Stein Vision Science Training Grant. G.H.T. is the Charles Kenneth Feldman and Jules and Doris Stein Research to Prevent Blindness Professor.
Abbreviations
- 11cRAL
11-cis-retinaldehyde
- 11cRDH
11-cis-retinol dehydrogenase
- 11cRE
11-cis-retinyl ester
- 11cROL
11-cis-retinol
- A2E
N-retinylidene-N-retinylethanolamine
- A2PE-H2
dihydro-N-retinylidene-N-retinylphosphatidylethanolamine
- atRAL
all-trans-retinaldehyde
- atROL
all-trans-retinol
- RPE
retinal pigment epithelium
- N-ret-PE
N-retinylidene-phosphatidylethanolamine
- ERG
electroretinography
- mAU
milliabsorption units
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 4353.
References
- 1.Leibowitz H M, Krueger D E, Maunder L R, Milton R C, Kini M M, Kahn H A, Nickerson R J, Pool J, Colton T L, Ganley J P, et al. Surv Ophthalmol. 1980;24:335–610. [PubMed] [Google Scholar]
- 2.Klein R, Klein B E, Linton K L P. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Lee B L, Heckenlively J R. In: Retina-Vitreous-Macula. Guyer D R, Yannuzzi L A, Chang S, Shields J A, Green W R, editors. Philadelphia: Saunders; 1999. pp. 978–988. [Google Scholar]
- 4.Blacharski P A. Fundus flavimaculutus. New York: Raven; 1988. [Google Scholar]
- 5.Eagle R C, Jr, Lucier A C, Bernardino V B, Jr, Yanoff M. Ophthalmology. 1980;87:1189–1200. doi: 10.1016/s0161-6420(80)35106-3. [DOI] [PubMed] [Google Scholar]
- 6.Birnbach C D, Jarvelainen M, Possin D E, Milam A H. Ophthalmology. 1994;101:1211–1219. doi: 10.1016/s0161-6420(13)31725-4. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg R H. Doc Ophthalmol. 1985;60:327–346. doi: 10.1007/BF00158922. [DOI] [PubMed] [Google Scholar]
- 8.Allikmets R, Shroyer N F, Singh N, Seddon J M, Lewis R A, Bernstein P S, Peiffer A, Zabriskie N A, Li Y, Hutchinson A, et al. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 9.Illing M, Molday L L, Molday R S. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 10.Azarian S M, Travis G H. FEBS Lett. 1997;409:247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- 11.Weng J, Mata N L, Azarian S M, Tzekov R T, Birch D G, Travis G H. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 12.Mata N L, Weng J, Travis G H. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mata N L, Tzekov R T, Liu X R, Weng J, Birch D G, Travis G H. Invest Ophthalmol Visual Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- 14.Sun H, Molday R S, Nathans J. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- 15.Ahn J, Wong J T, Molday R S. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- 16.Sakai N, Decatur J, Nakanishi K, Eldred G E. J Am Chem Soc. 1996;118:1559–1560. [Google Scholar]
- 17.Reinboth J J, Gautschi K, Munz K, Eldred G E, Reme C E. Exp Eye Res. 1997;65:639–643. doi: 10.1006/exer.1997.0367. [DOI] [PubMed] [Google Scholar]
- 18.Parish C A, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Proc Natl Acad Sci USA. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schutt F, Davies S, Kopitz J, Holz F G, Boulton M E. Invest Ophthalmol Visual Sci. 2000;41:2203–2208. [PubMed] [Google Scholar]
- 20.Suter M, Reme C, Grimm C, Wenzel A, Jaattela M, Esser P, Kociok N, Leist M, Richter C. J Biol Chem. 2000;275:39625–39630. doi: 10.1074/jbc.M007049200. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow J R, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Invest Ophthalmol Visual Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 22.Finnemann S C, Leung L W, Rodriguez-Boulan E. Proc Natl Acad Sci USA. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peck G L, Olsen T G, Yoder F W, Strauss J S, Downing D T, Pandya M, Butkus D, Arnaud-Battandier J. N Engl J Med. 1979;300:329–333. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- 24.Weleber R G, Denman S T, Hanifin J M, Cunningham W J. Arch Ophthalmol. 1986;104:831–837. doi: 10.1001/archopht.1986.01050180065031. [DOI] [PubMed] [Google Scholar]
- 25.Fraunfelder F T, Fraunfelder F W, Edwards R. Am J Ophthalmol. 2001;132:299–305. doi: 10.1016/s0002-9394(01)01024-8. [DOI] [PubMed] [Google Scholar]
- 26.Law W C, Rando R R. Biochem Biophys Res Commun. 1989;161:825–829. doi: 10.1016/0006-291x(89)92674-0. [DOI] [PubMed] [Google Scholar]
- 27.Gamble M V, Mata N L, Tsin A T, Mertz J R, Blaner W S. Biochim Biophys Acta. 2000;1476:3–8. doi: 10.1016/s0167-4838(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 28.Sieving P A, Chaudhry P, Kondo M, Provenzano M, Wu D, Carlson T J, Bush R A, Thompson D A. Proc Natl Acad Sci USA. 2001;98:1835–1840. doi: 10.1073/pnas.041606498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch H J, Rivest R W, Wurtman R J. Neuroendocrinology. 1980;31:106–111. doi: 10.1159/000123059. [DOI] [PubMed] [Google Scholar]
- 30.Nusinowitz S, Ridder W, Heckenlively J. In: Systemic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods. Smith R, John S W M, Nishina P, editors. Dordrecht, The Netherlands: Kluwer; 2001. pp. 320–344. [Google Scholar]
- 31.Hood D C, Birch D G. Vision Res. 1993;33:1605–1618. doi: 10.1016/0042-6989(93)90027-t. [DOI] [PubMed] [Google Scholar]
- 32.Hood D C, Birch D G. Invest Ophthalmol Visual Sci. 1990;31:2070–2081. [PubMed] [Google Scholar]
- 33.Suzuki T, Fujita Y, Noda Y, Miyata S. Vision Res. 1986;26:425–429. doi: 10.1016/0042-6989(86)90185-9. [DOI] [PubMed] [Google Scholar]
- 34.Mata N L, Radu R A, Clemmons R, Travis G H. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saari J C, Bredberg D L. J Biol Chem. 1987;262:7618–7622. [PubMed] [Google Scholar]
- 36.Mata N L, Tsin A T. Biochim Biophys Acta. 1998;1394:16–22. doi: 10.1016/s0005-2760(98)00078-2. [DOI] [PubMed] [Google Scholar]
- 37.Driessen C, Winkens H J, Hoffmann K, Kuhlmann L D, Janssen B P M, van Vugt A H M, Van Hooser J P, Wieringa B E, Deutman A F, Palczewski K, et al. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong S L, Liou G I, Landers R A, Alvarez R A, Bridges C D. J Biol Chem. 1984;259:6534–6542. [PubMed] [Google Scholar]
- 39.Palczewski K, Van Hooser J P, Garwin G G, Chen J, Liou G I, Saari J C. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- 40.Nau H. J Am Acad Dermatol. 2001;45:S183–S187. doi: 10.1067/mjd.2001.113720. [DOI] [PubMed] [Google Scholar]