Abstract
Atherosclerosis is the major cause of adult mortality in the developed world, and a significant contributor to atherosclerotic plaque progression involves smooth muscle cell recruitment to the intima of the vessel wall. Controversy currently exists on the exact origin of these recruited cells. Here we use sex-mismatched bone marrow transplant subjects to show that smooth muscle cells throughout the atherosclerotic vessel wall can derive from donor bone marrow. We demonstrate extensive recruitment of these cells in diseased compared with undiseased segments and exclude cell–cell fusion events as a cause for this enrichment. These data have broad implications for our understanding of the cellular components of human atherosclerotic plaque and provide a potentially novel target for future diagnostic and therapeutic strategies.
Keywords: precursor‖bone marrow‖plaque‖intima‖chimerism
Atherosclerosis is a complex disease, and our current understanding represents a synthesis of numerous hypotheses developed over the past century and a half (1–4). The hypotheses have been continually modified to take into account new experimental and clinical data (5–9). Currently, the pathogenesis of atherosclerosis is thought to involve a sequence of biologic events within the intima of the vessel wall which includes vascular injury, monocyte recruitment and macrophage formation, lipid deposition, platelet degranulation and thrombosis, and vascular smooth muscle cell migration, proliferation and extracellular matrix synthesis (9).
Until recently, the smooth muscle cell component of primary atherosclerotic plaque was thought to solely derive from the surrounding local vessel wall with migration of phenotypically distinct smooth muscle cells from the media through fenestrations in the internal elastic lamina to the intima where proliferation of these altered cells occurs (2, 7, 10). These latter assumptions have been questioned in the light of animal studies suggesting that bone marrow progenitor cells can infiltrate the atherosclerotic intima and differentiate in vivo to form smooth muscle cells within the plaque (11–14). We have also recently shown that phenotypically distinct smooth muscle cells can be grown in culture from human peripheral blood, suggesting the existence of a circulating smooth muscle progenitor (15). However, no in vivo data currently exist to support a bone marrow origin for smooth muscle cells within primary atherosclerotic plaque of human subjects.
To investigate whether smooth muscle cells participating in human atherosclerotic plaque derive from the bone marrow, we studied diseased and undiseased coronary artery segments of deceased subjects who had previously undergone gender-mismatched bone marrow transplantation (BMT) and were found to have coexistent coronary atherosclerosis at autopsy. Immunohistochemistry for smooth muscle specific markers was combined with flourescence in situ hybridization (FISH) for the X and Y chromosomes to discriminate male and female smooth muscle cells of donor and recipient origin in diseased coronary arteries. Control same-gender bone marrow transplant subjects were used to assess FISH sex chromosome discrimination and to detect vascular tissue evidence of background fetal and maternal microchimerism (16).
Methods
Patients and Autopsy Tissue.
Coronary artery specimens from 13 subjects (eight gender-mismatched subjects and five gender-matched control subjects) who received BMT were studied. All patients had survived 1 month or more after transplant with clinical report of engraftment. The institutional review board at Mayo Clinic approved the use of all autopsy specimens and all patients gave consent before transplant for their tissue to be used in research studies.
Combined Immunohistochemical and FISH Analysis.
Formalin-fixed, paraffin-embedded blocks were cut into 4-μm sections and placed on microscope slides. Multiple sections through diseased and undiseased coronary artery segments were obtained from each subject. The sections were deparaffinized by using CitriSolv (Fisher Scientific) and rehydrated in an ethanol series. Immunohistochemical analysis was performed by using monoclonal antibodies against smooth-muscle α-actin, myosin heavy chain, and calponin as described (15, 17). The secondary antibodies used were antimouse antibodies conjugated to Cy-3 (Molecular Probes) (red stain) or AlexaFluor488 (Molecular Probes) (green stain).
After immunostaining, FISH was immediately performed. The tissue was dehydrated twice in 100% ethanol for 1–2 min and then heated in a steamer in preheated 1 mM EDTA (pH 8.0) for 20 min, followed by 0.05 μg/μl proteinase K (Sigma) or pepsin A (2,100 units/mg) in buffer (0.05M Tris⋅HCl/2 mM CaCl2, pH 7.8/0.01 M EDTA/0.01 M NaCl) at 37°C for 15 min. The tissue was then rinsed in an ethanol series. Subsequently, the hybridization probe mixture (Vysis, 30-804824) was applied to the sections. The DNA probes used were specific for the α satellite regions of the X and Y chromosomes, and were fluorescently labeled. The X chromosome probe (CEP X, Vysis, B-7322) was labeled with cyanine-3 (red), and the Y chromosome probe (CEP Y, Vysis, B-6927) was labeled with fluorescein isothiocyanate (green). For combined immunostaining and FISH, Cy3 actin staining with Y chromosome, or Alexa Fluor actin staining with X chromosome combinations were used. In separate experiments a probe to the centromere of human chromosome 18 (CEP 18 SpectrumAqua-light blue-Vysis) was also combined with X and Y chromosome analysis to evaluate cell ploidy and exclude cell fusion. Ploidy analysis was performed on all gender-mismatched subjects.
Coverslips were affixed and sealed with rubber cement, and the slides were incubated at 80°C for 3 min to denature all DNA and then were incubated at 37°C overnight. After hybridization, the slides were washed in 2× SSC/0.1% Nonidet P-40 solution at room temperature, counterstained with 0.03 μg/ml 4′,6-diamidino-2-phenylindole (DAPI), and mounted with Vectashield (Vector Laboratories, H-1200). FISH signals were enumerated by using a Zeiss Axioplan microscope equipped with a triple-pass filter (Vysis).
Data and Statistical Analysis.
Sections were reviewed on the same microscope to ensure the images were consistent and reproducible. Only sections that contained clear morphology with adequate immunohistochemical and FISH staining were considered for analysis. Cells were considered smooth muscle if surrounded by smooth-muscle α-actin as described (18). A nucleus was considered of male origin if there was a clear green signal or female origin if there were two distinct red signals. A nucleus was considered diploid if there were two light blue signals.
The number of donor derived cells that were smooth muscle α-actin positive were expressed as a percentage of all smooth muscle cells counted in the intima, media, and in adventitial microvessels. The summation of counts from each subject was averaged within the intima, media and microvessels within the adventitia and expressed as percentages (±SD) of the total smooth muscle cell counts within these vascular regions. A total of 115,080 nuclei were analyzed, approximating 12,000 nuclei (8,000 diseased and 4,000 undiseased segments) per gender-mismatched subject (n = 8) and 3,600 nuclei (diseased and undiseased segments) per gender-matched control subject (n = 5). Approximately 16,000 nuclei were analyzed for chromosomal ploidy (1,000 nuclei per diseased and undiseased segment per gender-mismatched patient). A pathologist who was unaware of the age or sex of the patient independently assessed the histology of the coronary arteries to classify the presence or absence of arterial disease. Comparisons between diseased and undiseased groups were made by ANOVA, and P < 0.05 was considered significant.
Results
Characteristics of Study Subjects.
Thirteen autopsy subjects were studied, eight subjects (four male, four female) had undergone gender-mismatched and five control subjects (three male, two female) had undergone gender-matched BMT. The clinical characteristics of the subjects studied with regard to donor/recipient gender, baseline disease, conditioning regimen, time from BMT to death, and pathologic extent of coronary disease are shown in Table 1. The mean age (±SD) was 41 ± 10 years for males, 43 ± 11 years for females. The time from BMT to death ranged from 41 to 964 days in male recipients and from 91 to 1235 days in female recipients. One patient had chronic graft versus host disease (GVHD) documented, but there was no relationship between the presence or absence of GVHD and the level of donor smooth muscle cell chimerism detected in our study subjects. The pathologic extent of coronary artery disease was graded on a scale of 1–4 in each subject according to previous guidelines (19).
Table 1.
Clinical and pathologic characteristics of gender-mismatched and gender-matched (control) BMT subjects
| Patient no. | Gender [D] | Gender [R] | Age, years | Underlying disease | Conditioning regimen | Graft duration, days | Coronary pathology |
|---|---|---|---|---|---|---|---|
| Gender-mismatched bone marrow transplant subjects | |||||||
| 1 | Male | Female | 42 | Chronic lymphocytic leukemia | Cyclophosphamide TBI | 964 | Grade III fibrocellular plaque |
| 2 | Male | Female | 46 | Chronic granulocytic leukemia | Cyclophosphamide busulphan | 501 | Grade II fibrocellular plaque |
| 3 | Male | Female | 33 | Chronic granulocytic leukemia | Cyclophosphamide TBI | 41 | Grade III fibrocellular plaque |
| 4 | Male | Female | 30 | Myelodysplastic syndrome | Cyclophosphamide TBI | 90 | Diffuse intimal thickening |
| 5 | Female | Male | 39 | Myeloma | Cyclophosphamide busulphan | 1235 | Grade III fibrocellular plaque |
| 6 | Female | Male | 29 | Myelodysplastic syndrome | Cyclophosphamide TBI | 78 | Grade III fibrocellular plaque |
| 7 | Female | Male | 45 | Acute myelogenous leukemia | Cyclophosphamide TBI | 131 | Grade III fibrocalcific plaque |
| 8 | Female | Male | 58 | Myelofibrosis | Cyclophosphamide busulphan | 91 | Diffuse intimal thickening |
| Gender-matched bone marrow transplant subjects | |||||||
| 9 | Male | Male | 55 | Myelodysplastic syndrome | Cyclophosphamide TBI | 655 | Grade II fibrocellular plaque |
| 10 | Male | Male | 46 | Acute lymphocytic leukemia | Cyclophosphamide TBI | 527 | Grade III fibrocellular plaque |
| 11 | Male | Male | 45 | Myelodysplastic syndrome | Cyclophosphamide TBI | 153 | Diffuse intimal thickening |
| 12 | Female | Female | 46 | Chronic granulocytic leukemia | Cyclophosphamide busulphan | 108 | Diffuse intimal thickening |
| 13 | Female | Female | 52 | Chronic granulocytic leukemia | Cyclophosphamide TBI | 901 | Grade II fibrolipid plaque |
[D] and [R] indicate donor and recipient, respectively. TBI, total body irradiation.
Detection of Donor Smooth Muscle Cells in Diseased and Undiseased Vessel Wall of Gender-Mismatched BMT Subjects.
Eight subjects who had undergone gender-mismatched BMT (four male to female; four female to male) were studied with combined smooth muscle marker immunohistochemistry and FISH. The results of sex chromosome identification and smooth muscle marker coimmunostaining of diseased and undiseased coronary artery segments from these subjects are shown in Table 2.
Table 2.
Percentage of smooth muscle cells positive for donor sex chromosome markers, indicating bone marrow origin, in diseased and undiseased vessels from the same gender-mismatched BMT subjects
| [D]=>[R] gender | Patient no. | Diseased vessels, % [D] SMC
|
Undiseased vessels, % [D] SMC
|
||||
|---|---|---|---|---|---|---|---|
| Intima | Media | Advent. MV | Intima | Media | Advent. MV | ||
| M → F FISH probe Y | 1 | 4.3 ± 1.8 | 2.5 ± 0.6 | 5.7 ± 1.6 | 0.14 ± 0.07 | 0.08 ± 0.03 | 0.12 ± 0.04 |
| 2 | 9.4 ± 2.9 | 2.2 ± 0.8 | 5.2 ± 0.9 | 0.10 ± 0.01 | 0.05 ± 0.02 | 0.11 ± 0.02 | |
| 3 | 10.2 ± 1.9 | 1.7 ± 0.8 | 10.7 ± 2.1 | 0.12 ± 0.09 | 0.14 ± 0.05 | 0.15 ± 0.04 | |
| 4 | 10.1 ± 2.1 | 2.5 ± 0.9 | 4.3 ± 0.6 | 0.08 ± 0.01 | 0.11 ± 0.03 | 0.11 ± 0.03 | |
| Mean ± SD | 9.4 ± 2.8* | 2.2 ± 0.9* | 6.5 ± 2.9* | 0.11 ± 0.03 | 0.10 ± 0.04 | 0.13 ± 0.02 | |
| F → M FISH probe XX | 5 | 9.7 ± 3.1 | 1.8 ± 1.1 | 6.6 ± 1.2 | 0.13 ± 0.05 | 0.15 ± 0.04 | 0.20 ± 0.01 |
| 6 | 11.4 ± 4.2 | 2.1 ± 1.3 | 11.4 ± 3.7 | 0.09 ± 0.02 | 0.09 ± 0.04 | 0.17 ± 0.02 | |
| 7 | 9.9 ± 2.3 | 1.9 ± 1.3 | 1.9 ± 0.4 | 0.11 ± 0.06 | 0.12 ± 0.07 | 0.15 ± 0.04 | |
| 8 | 10.9 ± 5.1 | 1.1 ± 0.7 | 3.5 ± 1.5 | 0.10 ± 0.02 | 0.07 ± 0.02 | 0.13 ± 0.02 | |
| Mean ± SD | 10.8 ± 3.3* | 1.7 ± 1.1* | 5.9 ± 4.2* | 0.11 ± 0.02 | 0.11 ± 0.03 | 0.16 ± 0.03 | |
SMC indicates smooth muscle cells determined by immunofluorescence, [D] and [R] denote donor and recipient subjects, respectively, and Advent. MV denotes adventitial microvessels. M → F and F → M indicate male to female and female to male BMT.
P < 0.001 for diseased segments compared to undiseased segments in the coronary artery specimens of the same subjects.
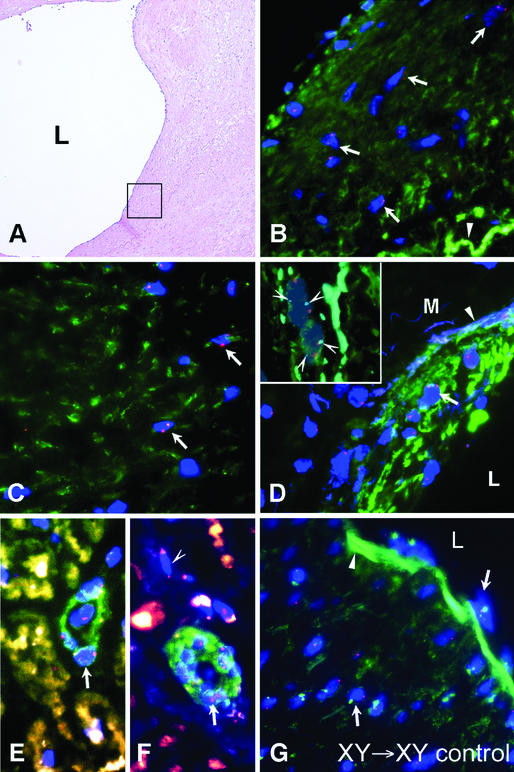
In female subjects who received a male BMT the mean percentage of male smooth muscle cells within the intima, media and adventitial microvessels of diseased coronary artery segments was 9.4 ± 2.8%, 2.2 ± 0.9%, and 6.5 ± 2.9%, respectively (Fig. 1 A–H, Table 2). This was ≈100-fold higher than the mean percentage of donor smooth muscle cells seen in the same layers of undiseased coronary artery segments from the same subjects (0.11 ± 0.03%, 0.1 ± 0.04%, and 0.13 ± 0.02%, P < 0.001, Fig. 2 A and C, and Table 2). In diseased vessel segments male donor smooth muscle cells were detected throughout the fibrocellular subendothelial intima (Fig. 1 A and B), deep intima (Fig. 1 C, E, and F), media (Fig. 1D). Male smooth muscle cells were also detected in microvessels of varying size within the adventitia of these female arteries (Fig. 1 G and H). In undiseased control segments male smooth muscle cells were rarely detected in any layer of the vessel wall (Fig. 2 A and C).
Figure 1.
Intimal atherosclerotic plaque, media, and adventitial microvessels from female recipients (subjects 1–4, Table 1) who received a male BMT (XY → XX; A–H). (A) Hematoxylin and eosin staining showing a grade III atherosclerotic plaque from subject 1. L, lumen; filled arrowhead, internal elastic lamina. The two boxes indicate the subendothelial intima and the deep intima that are subsequently analyzed with dual α smooth muscle actin staining and FISH for Y chromosome in B and C. (B) Combined immunostaining for α smooth muscle actin and FISH for Y chromosome showing clusters of male cells (blue DAPI-stained nuclei with green dot) surrounded by positive α smooth muscle actin staining (red Cy-3 stain surrounding male nuclei, white arrows) in the subendothelial intima of plaque from subject 1. A row of white arrows point to a cluster of male actin-positive cells. Some actin staining cells were negative for the Y chromosome (open arrowhead). (C) Male smooth muscle cells deeper within the intima of plaque from subject 1 colabeling for α smooth muscle actin and the Y chromosome (white arrows). (D) Male smooth muscle cells in the media of coronary artery from subject 2 showing combined labeling for α smooth muscle actin and the Y chromosome (white arrows). The open arrowhead indicates the elastic lamellae of the media (M) and the filled arrowhead indicates the internal elastic lamina. (Inset) Chromosomal multiploidy analysis of male donor smooth muscle cells in female coronary artery showing diploid nature and lack of cell fusion. Shown are two separate nuclei (DAPI) each showing single X chromosome (large red dot) and Y chromosome (green dot) and also two chromosome 18 markers per nucleus (light blue dots, arrowheads), indicating each nucleus is diploid. The background red staining indicates α smooth muscle actin staining with Cy-3 fluorescence. (E and F) Male smooth muscle cells within the subendothelial and deep intima of a coronary artery plaque from subject 4 showing combined labeling for smooth muscle myosin heavy chain (E, red staining) and smooth muscle calponin (F, red staining) and the Y chromosome (white arrows). (G and H) Male smooth muscle cells in microvessels of the adventitia of an atherosclerotic coronary artery from subject 3 showing combined labeling for α smooth muscle actin and the Y chromosome (white arrows). The open arrowhead indicates an actin staining cell that was negative for the Y chromosome. (I) Combined FISH for X and Y chromsome and α smooth muscle actin costaining showing absence of male cells in a control diseased artery of a same-gender female BMT subject. In B–I the nuclei were counterstained with DAPI (blue).
Figure 2.
Infrequent donor cells detected in the artery wall of undiseased vessel segments from male and female subjects with gender-mismatched BMT. (A) Hematoxylin and eosin staining of undiseased vessel segment from female recipient who had gender-mismatched BMT. The box indicates an area of intima and media and adventitia subsequently analyzed in C by using α smooth muscle actin-Cy3 staining and FISH for X and Y chromosomes. (B) Hematoxylin and eosin staining of undiseased branch vessel segment from male subject who had gender-mismatched BMT. The box indicates an area of the intima and media subsequently analyzed in D by using α smooth muscle actin-AlexaFluor staining and FISH for the X and Y chromosomes. (C) Lack of male smooth muscle cells in the wall of an undiseased vessel segment in same female subject as in A. Note multiple XX chromosome positive female nuclei (open arrowheads) throughout the vessel and a solitary Y chromosome positive nucleus (arrow) in the adventitia. (D) Infrequent female donor smooth muscle cells in the intima and media of an undiseased vessel segment in the same male subject as in B. Note single XX positive nucleus (two red dots, arrow) in the media where multiple XY positive (red and green dot) male cells are present. In all four panels, an arrowhead indicates the internal elastic lamina.
In male subjects who received a female BMT the mean percentage of female smooth muscle cells within the intima, media and adventitial microvessels of diseased coronary artery was 10.8 ± 3.3%, 1.7 ± 1.1%, and 5.9 ± 4.2%, respectively (Fig. 3, Table 2). This was also ≈100-fold greater than the percentages seen in the undiseased segments from the same subjects (0.11 ± 0.02%, 0.11 ± 0.03%, 0.16 ± 0.03%, P < 0.001, Fig. 3 B and D, and Table 2). In diseased segments donor XX positive smooth muscle cells were detected in the shoulder region of male fibrocellular plaque (Fig. 3 A and B), deep within the intima (Fig. 3C), in diffuse intimal thickenings (Fig. 3D), and within adventitial microvessels (Fig. 3 E and F). In undiseased segments female smooth muscle cells were rarely detected in the vessel wall (Fig. 3 B and D).
Figure 3.
Intimal atherosclerotic plaque, media, diffuse intimal thickening and adventitial microvessels from male recipients (subjects 5–8, Table 1) who received a female BMT (XX → XY; A–F). (A) Hematoxylin and eosin staining showing a grade III atherosclerotic plaque from subject 5. L indicates the lumen and the box indicates the subendothelial intima subsequently analyzed in B with dual α smooth muscle actin staining and FISH for the X chromosome. (B) Combined immunostaining for α smooth muscle actin and FISH for the XX chromosome showing female cells (blue nuclei with two red dots) surrounded by positive α smooth muscle actin staining (green AlexaFluor staining surrounding female nuclei; white arrows) scattered throughout the subendothelial intima of plaque from subject 5. The arrowhead indicates the internal elastic lamina. (C) Female smooth muscle cells deeper within the intima of plaque from subject 6 colabeling for α smooth muscle actin and the XX chromosome (white arrows). (D) Female smooth muscle cells within the intima of a coronary artery with diffuse intimal thickening from subject 8 showing combined labeling for α smooth muscle actin and the XX chromosome (white arrow). L indicates the lumen and the arrowhead indicates the internal elastic lamina. (Inset) Chromosomal multiploidy analysis of female donor smooth muscle cells in male coronary artery showing diploid nature. Two separate female nuclei (DAPI), each showing XX chromosomes (two red dots per cell), no Y chromosome, and two chromosome 18 markers (light blue dots, arrowheads) per nucleus indicating diploid status. The green staining surrounding both nuclei indicates α smooth muscle actin labeling with AlexaFluor-conjugated antibody. (E and F) Female smooth muscle cells in microvessels of the adventitia of an atherosclerotic coronary artery from subject 7 showing combined labeling for α smooth muscle actin and the XX chromosome (white arrows). The open arrowhead indicates a female cell not costaining for actin. (G) Control male recipient of same-gender BMT showing absence of female (XX) cells in the intima and media of the vessel wall. Arrows indicate male nuclei and a filled arrowhead indicates the internal elastic lamina. In B–G the nuclei were counterstained with DAPI (blue).
XY Chromosome FISH Detection Efficiency and Lack of Evidence for Cell Fusion Events or Background Feto-Maternal Microchimerism.
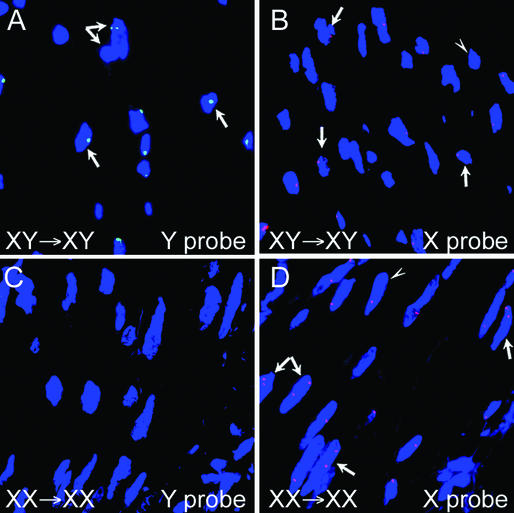
Sections of coronary arteries from five same-gender BMT subjects were initially used as controls for FISH identification of X and Y chromosomes, respectively. These sections also acted as controls to determine the level of fetal and maternal microchimerism in vascular tissue (i.e., percentage of opposite gender cells in male and female vascular tissue). The sections from the male recipients who received male donor marrow showed Y chromosome positivity in 45.9 ± 7.8% of cells within the intima, media and adventitial microvessels and no XX positive cells (Fig. 4 A and B). In the case of the control female recipients who received female donor marrow, no Y chromosome was detected in the intima, media, or adventitial microvessels, and 42.2 ± 6% of cells within these layers were XX chromosome positive (Fig. 4 C and D). This FISH detection efficiency was consistent with previous FISH studies of tissue sections (20). Moreover, when using the combined X and Y probes with smooth muscle marker detection, no evidence of opposite gender microchimerism was detected in any vascular tissue from the five control subjects (≈18,000 nuclei analyzed) (Figs. 1I and 2G).
Figure 4.
No chimeric cells are detected in the vessel wall of same-gender BMT subjects. (A) FISH for male cells showing Y chromosome body (green dot in blue nuclei) in coronary atherosclerotic plaque from a male subject who had same-gender BMT. The arrows indicate male cells with the dual arrow showing two overlapping male nuclei. (B) FISH for female cells in coronary atherosclerotic plaque from the same male subject as in A showing single X chromosome (red dot in nuclei) in each of the intimal cells. The arrows indicate the single X chromosome cells and the arrowhead indicates a cell negative for the X chromosome. No XX chromosome was detected in cells of this subject. (C) FISH for male cells in media of diffuse intimal thickening from a female subject who had same-gender BMT showing lack of Y chromosome labeling. (D) FISH for female cells showing XX chromosome labeling (two red dots per nucleus; arrows) in cells in the media of diffuse intimal thickening from same female subject as in C. The presence of single red dots in some nuclei (arrowhead) indicates that not all cells labeled positive for the XX chromosome.
To exclude the possibility that recipient smooth muscle cells fused with donor bone marrow (21), chromosomal multiploidy analysis was performed by using chromosome 18 (Ch.18) as an additional probe to identify diploid or tetraploid cells. Combined FISH analysis of X, Y, and Ch.18 in nuclei coimmunostaining for α smooth muscle actin confirmed universal diploid cell status, and no evidence of cell fusion in either male or female gender-mismatched scenarios (Figs. 1D and 2D Insets).
Discussion
This study shows that, after BMT, smooth muscle cells of donor origin are markedly enriched in coronary atherosclerotic plaque compared with the undiseased vessel wall. These data validate, in human subjects, a progenitor cell paradigm for atherosclerosis development recently described in experimental animal models. Our experimental design used multiple smooth-muscle-cell-specific markers to identify only differentiated smooth muscle cells. We also excluded other potential confounders for significant chimerism such as donor leukocyte-recipient smooth muscle cell fusion events and feto-maternal microchimerism by using chromosomal multiploidy analysis and same-gender-matched control subjects, respectively.
The accepted paradigm for atherogenesis involves migration of medial smooth muscle cells through fenestrations in the internal elastic lamina to the intima in response to injury, with subsequent proliferation and extracellular matrix production by these cells contributing to plaque volume (2, 8, 9, 22). However, despite extensive investigation the exact origin of these intimal smooth muscle cells (dedifferentiated medial smooth muscle cell, smooth muscle progenitor cell, or myofibroblast) remains uncertain (23). Recent studies (20, 24) using tissue sections of gender-mismatched cardiac transplant subjects have suggested a progenitor origin for chimeric smooth muscle cells detected in epicardial and intramyocardial vessels. Whether such smooth muscle progenitor cells are of bone marrow origin remains unsettled, and the contribution of these cells to primary atherosclerosis is currently unknown.
The current study provides human data to support a circulating precursor origin for smooth muscle cells in primary atheroscleroisis (25, 26) and extends previous concepts on human smooth muscle cell plasticity and heterogeneity to in vivo disease. Because aspirated marrow contains a considerable amount of peripheral blood, we cannot be certain that the precursor cells actually come from the marrow. The exact phenotype of these transplantation-derived smooth muscle cells remains to be determined, but possibilities include circulating progenitors of a lineage-restricted mesenchymal or angioblastic phenotype (27), progenitors of hematopoietic origin (28, 29), or a multipotent stem cell within the transplanted bone marrow (30, 31).
Differential seeding of donor smooth muscle cells in diseased versus non diseased vessel wall segments in our study raises the possibility of a functional role for bone marrow precursor cells in atherogenesis. Moreover, marked enrichment of precursor smooth muscle cells in diseased artery segments as early as 41 days after BMT suggests specific homing of these cells to preexisting areas of atherosclerosis. The recruitment signal for these cells may be inflammation within preformed plaque that is known to potentiate vascular infiltration of marrow-derived leukocytes (32–34). The atherosclerotic vessel wall contains a range of adhesion molecules (35, 36), inflammatory cytokines, and growth factors such as platelet-derived growth factor (23), which may permit homing, attachment and subsequent differentiation of marrow derived cells into smooth muscle cells within plaque (31, 37, 38). Alternatively smooth muscle cell differentiation may occur in the circulation or bone marrow before homing of these cells to the diseased vessel wall.
Regardless of the specific homing and differentiation pathway, such vessel wall recruitment of smooth muscle precursors could potentiate expansion of plaque volume through cellular accumulation and extracellular matrix production within the intima. In this way an interplay between an inflammatory vessel wall and blood borne precursors may contribute to plaque progression. What is currently unknown is whether bone marrow derived smooth muscle cells possess a more proliferative and matrix secretory phenotype than native quiescent vascular smooth muscle cells, which could make them more atherogenic. It is interesting to note that similar levels of donor smooth muscle cell recruitment occurred in the intimal and adventitial compartments of diseased artery segments in our study. Because adventitial microvessel proliferation is known to closely correlate with plaque formation following vascular injury (39, 40), it is conceivable that smooth muscle precursors may contribute to adventitial microvessel formation and intimal expansion both intrinsic components of atherogenesis.
A protective function can also be speculated for these precursor cells in atherosclerosis. If an artery is injured, some smooth muscle cells must be recruited to repair that injury, while contractile function of the vessel wall is simultaneously maintained (41). It is known from animal studies that progenitor cells are implicated in vessel wall repair after vascular injury (11, 13), and it is therefore possible that precursor smooth muscle cells may also participate in intimal repair in human atherosclerosis. This repair function may include increased precursor smooth muscle cell recruitment to the fibrocellular portion of plaque, thereby augmenting fibrous cap strength and plaque stability. For example this could have protective clinical consequences if such cells were recruited to vulnerable atherosclerotic plaque. However, further work will be required to fully elucidate the specific protein expression profile and pro-atherogenic versus protective function of these cells in human plaque.
The similar levels of donor smooth muscle cell recruitment in the vessel wall seen at all times after transplantation is consistent with chimerism studies (20) of other cell types and supportive of stable chimerism in circulating progenitor cells of mesenchymal or hematopoietic origin (42, 43). Maintenance of stable progenitor cell numbers over time may allow continuous accumulation in established atherosclerotic plaque. It is also conceivable that vascular injury induced by the conditioning regimen in our subjects allowed niche recruitment of cells at the time of bone marrow infusion with early progenitor colonization of the injured vasculature. However, the segmental nature of vascular recruitment seen in our study suggests that the atherosclerotic vessel alone acts as a powerful homing source for these cells. What remains unknown from our current study is whether these precursor cells can also contribute to de novo plaque formation in the absence of preexisting disease.
In conclusion, the enrichment of donor smooth muscle cells, derived from transplanted bone marrow, in atherosclerotic vessel segments in our study is sufficient to warrant substantive future investigation into the role of these cells in vascular disease. It is important to highlight that the current study is a relative “snap-shot” (41 days to 4 years) compared with the time taken for occlusive human plaque to develop (decades). Therefore, the true contribution of bone marrow-derived cells to smooth muscle biology in adult plaque may be significantly underestimated in our study because we cannot detect precursor colonization of plaque that occurred before transplantation. Moreover, we also cannot estimate the expansion capability of these cells over the lifetime of the plaque. Finally, this study has implications for future strategies used to investigate and treat atherosclerosis. Such strategies might include targeting of progenitor cells in the circulation of diseased subjects to ameliorate vascular disease.
Acknowledgments
This work was supported by National Institutes of Health Grant HL66958-NMC, the Rappaport Program in Vascular Biology, and the Mayo Foundation.
Abbreviations
- BMT
bone marrow transplantation
- FISH
fluorescence in situ hybridization
- DAPI
4′,6-diamidino-2-phenylindole
References
- 1.Duguid J B, Robertson W B. Lancet. 1957;1:1205–1209. doi: 10.1016/s0140-6736(57)91786-5. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Glomset J A. Science. 1973;180:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- 3.Virchow R. Wien Med Wochenschr. 1856;6:825–829. [Google Scholar]
- 4.von Rokitansky C. A Manual of Pathological Anatomy. Berlin: Berlin Sydenham Society; 1852. [Google Scholar]
- 5.Ross R, Glomset J A. N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Arteriosclerosis. 1981;1:293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- 7.Ross R, Wight T N, Strandness E, Thiele B. Am J Pathol. 1984;114:79–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Ross R. N Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 10.Ueda M, Becker A E, Tsukada T, Numano F, Fujimoto T. Circulation. 1991;83:1327–1332. doi: 10.1161/01.cir.83.4.1327. [DOI] [PubMed] [Google Scholar]
- 11.Han C I, Campbell G R, Campbell J H. J Vasc Res. 2001;38:113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 12.Hillebrands J L, Klatter F A, van den Hurk B M, Popa E R, Nieuwenhuis P, Rozing J. J Clin Invest. 2001;107:1411–1422. doi: 10.1172/JCI10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell R N. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 15.Simper D, Stalboerger P G, Panetta C J, Wang S, Caplice N M. Circulation. 2002;106:1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 16.Nelson J L. Trends Mol Med. 2002;8:109–113. doi: 10.1016/s1471-4914(01)02269-9. [DOI] [PubMed] [Google Scholar]
- 17.Caplice N M, Mueske C S, Kleppe L S, Simari R D. Circulation. 1998;98:1051–1057. doi: 10.1161/01.cir.98.11.1051. [DOI] [PubMed] [Google Scholar]
- 18.Grimm P C, Nickerson P, Jeffery J, Savani R C, Gough J, McKenna R M, Stern E, Rush D N. N Engl J Med. 2001;345:93–97. doi: 10.1056/NEJM200107123450203. [DOI] [PubMed] [Google Scholar]
- 19.Edwards W D. In: Acute Myocardial Infarction. Gersh B J, Rahimtoola S H, editors. New York: Chapman and Hall; 1997. pp. 16–50. [Google Scholar]
- 20.Quaini F, Urbanek K, Beltrami A P, Finato N, Beltrami C A, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 21.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz D M, Nakano Y, Meyer E M, Morel L, Petersen B E, Scott E W. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 22.Stary H C. Eur Heart J. 1990;11,(Suppl. E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- 23.Berk B C. Physiol Rev. 2001;81:999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 24.Glaser R, Lu M M, Narula N, Epstein J A. Circulation. 2002;106:17–19. doi: 10.1161/01.cir.0000021923.58307.8f. [DOI] [PubMed] [Google Scholar]
- 25.Frid M G, Aldashev A A, Dempsey E C, Stenmark K R. Circ Res. 1997;81:940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- 26.Frid M G, Aldashev A A, Nemenoff R A, Higashito R, Westcott J Y, Stenmark K R. Arterioscler Thromb Vasc Biol. 1999;19:2884–2893. doi: 10.1161/01.atv.19.12.2884. [DOI] [PubMed] [Google Scholar]
- 27.Korbling M, Katz R L, Khanna A, Ruifrok A C, Rondon G, Albitar M, Champlin R E, Estrov Z. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 28.Jackson K A, Majka S M, Wang H, Pocius J, Hartley C J, Majesky M W, Entman M L, Michael L H, Hirschi K K, Goodell M A. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson S M, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine D M, et al. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Jahagirdar B N, Reinhardt R L, Schwartz R E, Keene C D, Ortiz-Gonzalez X R, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 31.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie C M. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 32.Berliner J A, Territo M, Almada L, Carter A, Shafonsky E, Fogelman A M. Arteriosclerosis. 1986;6:254–258. doi: 10.1161/01.atv.6.3.254. [DOI] [PubMed] [Google Scholar]
- 33.Libby P, Ridker P M, Maseri A. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 34.Patarroyo M, Prieto J, Beatty P G, Clark E A, Gahmberg C G. Cell Immunol. 1988;113:278–289. doi: 10.1016/0008-8749(88)90027-5. [DOI] [PubMed] [Google Scholar]
- 35.Huo Y, Ley K. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 36.Plutzky J. Am J Cardiol. 2001;88:10K–15K. doi: 10.1016/s0002-9149(01)01924-5. [DOI] [PubMed] [Google Scholar]
- 37.Hellstrom M, Kaln M, Lindahl P, Abramsson A, Betsholtz C. Development (Cambridge, UK) 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 39.Kumamoto M, Nakashima Y, Sueishi K. Hum Pathol. 1995;26:450–456. doi: 10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 40.Westerband A, Mills J L, Marek J M, Heimark R L, Hunter G C, Williams S K. J Vasc Surg. 1997;25:64–73. doi: 10.1016/s0741-5214(97)70322-7. [DOI] [PubMed] [Google Scholar]
- 41.Owens G K. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 42.Cilloni D, Carlo-Stella C, Falzetti F, Sammarelli G, Regazzi E, Colla S, Rizzoli V, Aversa F, Martelli M F, Tabilio A. Blood. 2000;96:3637–3643. [PubMed] [Google Scholar]
- 43.Nikolic B, Sykes M. Immuol Res. 1997;16:217–228. doi: 10.1007/BF02786391. [DOI] [PubMed] [Google Scholar]