Abstract
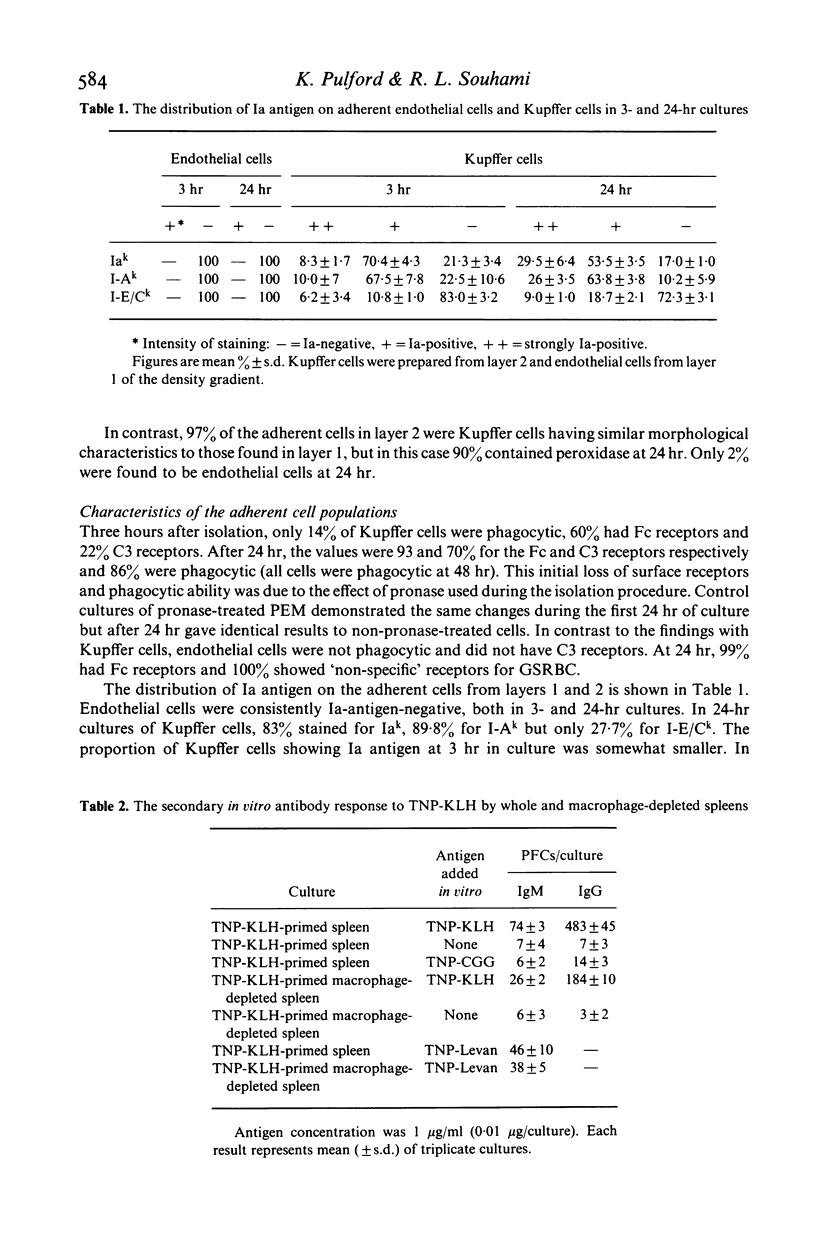
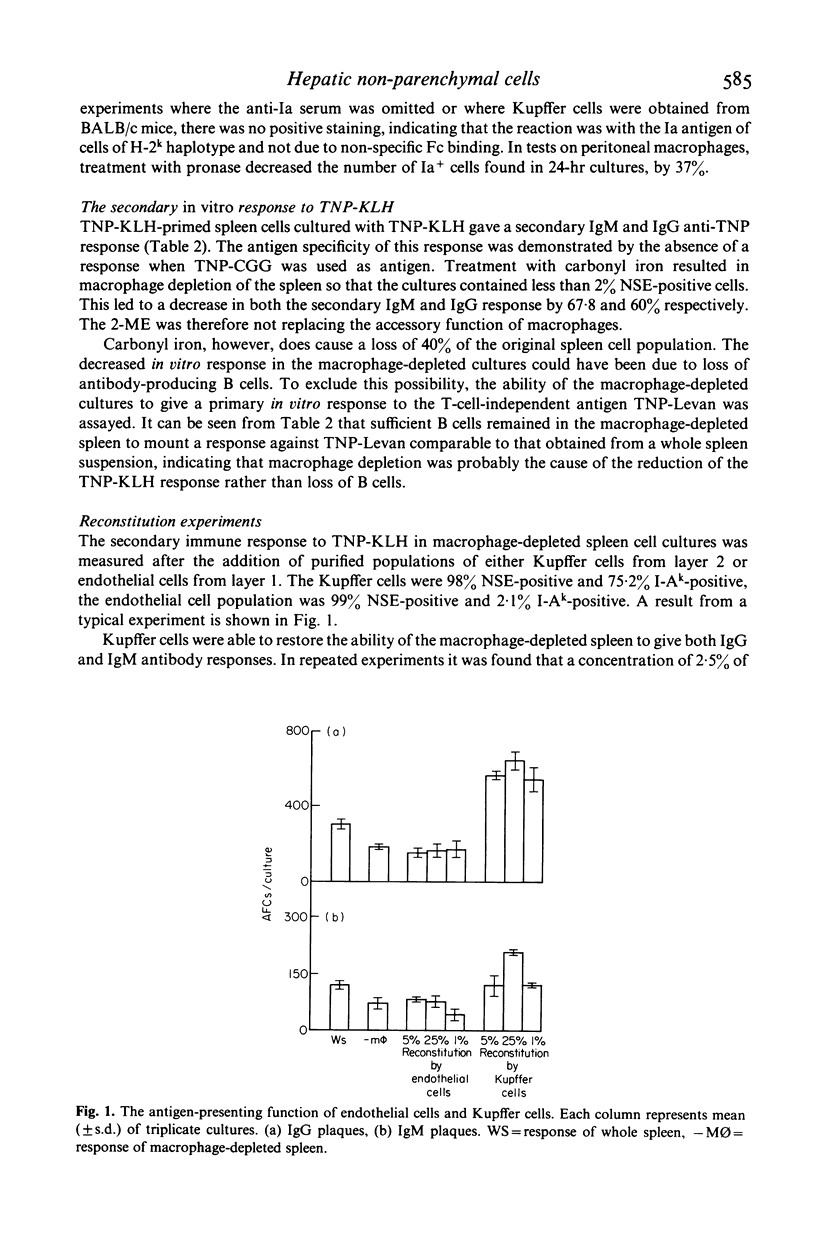
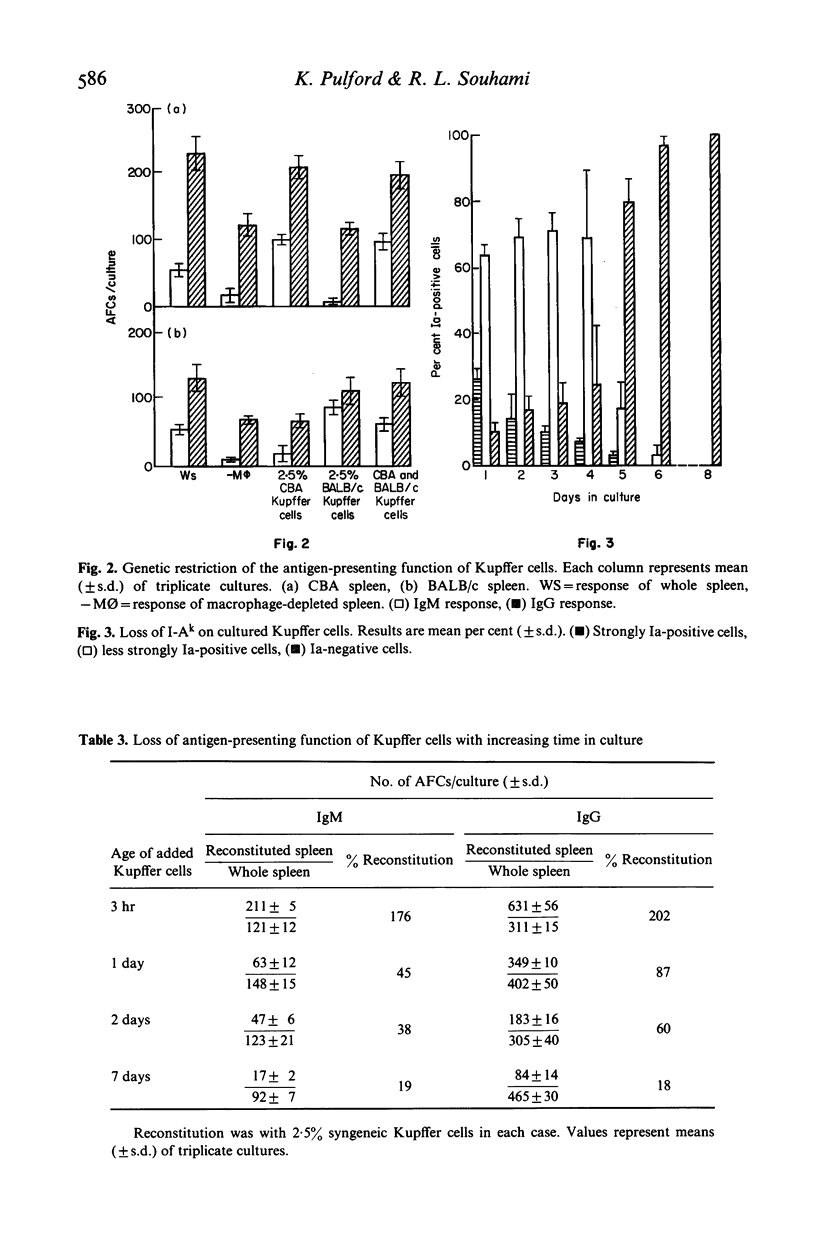
Hepatic endothelial cells and Kupffer cells have been isolated after pronase digestion of mouse liver and separated by density-gradient centrifugation on Percoll. The endothelial cells had a central round nucleus with a pale cytoplasm which contained non-specific esterase. They had Fc receptors but no Ia antigen, C3 receptor or phagocytic ability. In contrast, the phagocytic Kupffer cell had an initial rounded appearance and possessed Fc and C3 receptors and Ia antigens. Kupffer cells were capable of functioning as antigen-presenting cells as shown by their ability to reconstitute a secondary in vitro antibody in spleen cell cultures depleted of adherent cells. This function was genetically restricted. There was progressive loss of antigen-presenting function of Kupffer cells as time in culture increased. This appeared to be related to the loss of Ia antigen. Hepatic endothelial cells did not restore the antibody response in macrophage-depleted spleen cell cultures. The experiments show that Kupffer cells can function as antigen-presenting cells in a secondary antibody response in vitro and that this function is not due to the presence of endothelial cells in the isolated sinus lining cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Ahmann G. B., Sachs D. H., Hodes R. J. Genetic analysis of Ia determinants expressed on Con A-reactive cells. J Immunol. 1978 Jul;121(1):159–165. [PubMed] [Google Scholar]
- Bradfield J. W. Control of spillover. The importance of Kupffer-cell function in clinical medicine. Lancet. 1974 Oct 12;2(7885):883–886. doi: 10.1016/s0140-6736(74)91213-6. [DOI] [PubMed] [Google Scholar]
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H., Bergh O. J., Thorsby E. Antigen-presenting properties of human vascular endothelial cells. J Exp Med. 1980 Aug 1;152(2 Pt 2):249s–255s. [PubMed] [Google Scholar]
- Hirschberg H., Moen T., Thorsby E. Complement and cell-mediated specific destruction of human endothelial cells treated with anti-DRw antisera. Transplant Proc. 1979 Mar;11(1):776–778. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Moraes J. R., Stastny P. A new antigen system expressed in human endothelial cells. J Clin Invest. 1977 Aug;60(2):449–454. doi: 10.1172/JCI108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K., Souhami R. L. Cell division and giant cell formation in Kupffer cell cultures. Clin Exp Immunol. 1980 Oct;42(1):67–76. [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- Richman L. K., Klingenstein R. J., Richman J. A., Strober W., Berzofsky J. A. The murine Kupffer cell. I. Characterization of the cell serving accessory function in antigen-specific T cell proliferation. J Immunol. 1979 Dec;123(6):2602–2609. [PubMed] [Google Scholar]
- Richman L. R., Strober W., Berzofsky J. A. Genetic control of the immune response to myoglobin. III. Determinant-specific, two Ir gene phenotype is regulated by the genotype of reconstituting Kupffer cells. J Immunol. 1980 Feb;124(2):619–625. [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Antigen presentation by isolated guinea pig Kupffer cells. J Immunol. 1980 Apr;124(4):1740–1744. [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Characterization of isolated guinea pig Kupffer cells: accessory cell function in mitogen-induced T lymphocyte activation. J Immunol. 1979 Nov;123(5):1920–1927. [PubMed] [Google Scholar]
- Sjöberg O., Andersson J., Möller G. Requirement for adherent cells in the primary and secondary immune response in vitro. Eur J Immunol. 1972 Apr;2(2):123–126. doi: 10.1002/eji.1830020206. [DOI] [PubMed] [Google Scholar]
- Souhami R. L., Addison I. E., Bradfield J. W. Increased antibody production following depression of hepatic phagocytosis. Clin Exp Immunol. 1975 Apr;20(1):155–159. [PMC free article] [PubMed] [Google Scholar]
- Souhami R. L. The effect of colloidal carbon on the organ distribution of sheep red cells and the immune response. Immunology. 1972 Apr;22(4):685–694. [PMC free article] [PubMed] [Google Scholar]


