Abstract
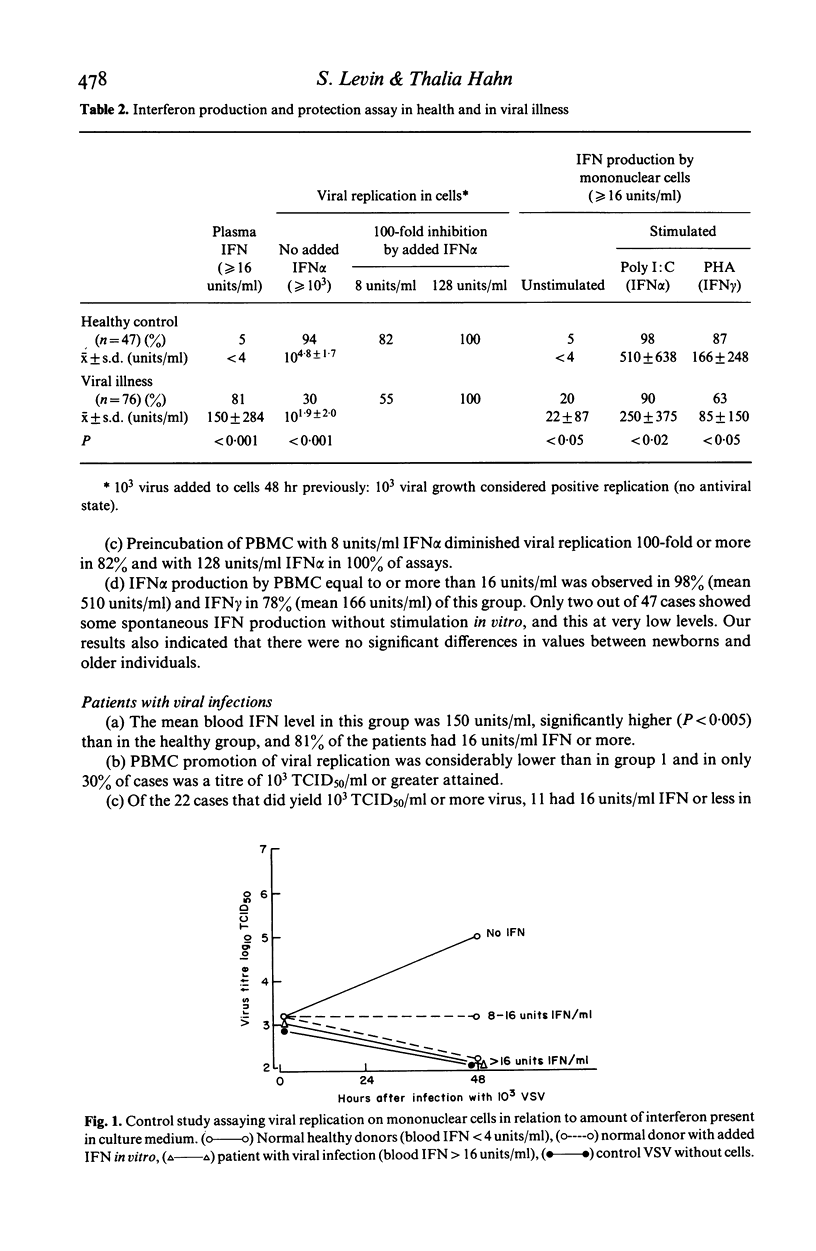
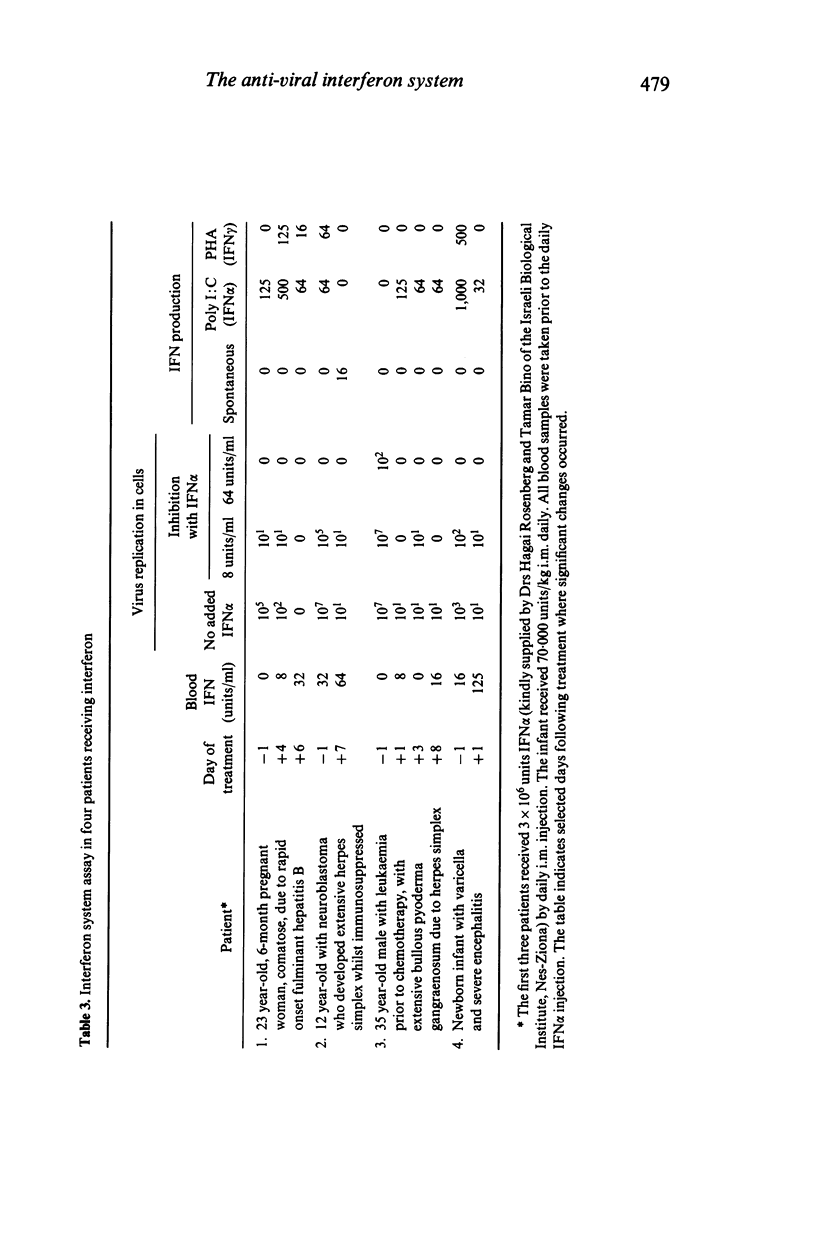
The interferon (IFN) system in man regulates viral replication, cell multiplication and immune functions. Its action against viruses takes place in two stages. The first is the production of IFN by cells following stimulation by a variety of IFN inducers including viruses, and the second is the action of this IFN on other cells inducing in them an antiviral state which prevents replication of infecting viruses. A series of assays is described which evaluates these different parameters of the antiviral IFN system. An attempt was made to correlate between IFN production and cell response to IFN on the one hand, and clinical status on the other. Results show that healthy persons have little or no IFN in the blood (mean less than 4 units/ml), and that in 94% their PBMC are not in an antiviral state. On the other hand, patients with acute viral diseases have significantly increased levels of IFN in their blood (mean 150 +/- 284 units/ml), and in 70% their cells are in an antiviral state. In some seriously ill patients with viral disease, the IFN system was found to be functionally deficient and treatment with human leucocyte IFN rapidly changed this. It is concluded that the examination of several biological parameters of the IFN system including the intracellular antiviral state induced by IFN is necessary in order to better evaluate this antiviral system. This will enable the clinician to obtain optimal pharmacokinetic information for determining which cases are most likely to respond to IFN therapy, and help to monitor the efficacy of this treatment.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron S., Dianzani F. General considerations of the interferon system. Tex Rep Biol Med. 1977;35:1–10. [PubMed] [Google Scholar]
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Denman A. M., Pelton B. K., Appleford D., Kinsley M. Virus infections of lympho-reticular cells and auto-immune diseases. Transplant Rev. 1976;31:79–115. [PubMed] [Google Scholar]
- Dunnick J. K., Galasso G. J. Clinical trials with exogenous interferon: summary of a meeting. J Infect Dis. 1979 Jan;139(1):109–123. doi: 10.1093/infdis/139.1.109. [DOI] [PubMed] [Google Scholar]
- Hahn T., Levin S., Handzel Z. T. Production of immune and viral interferon by lymphocytes of newborn infants. Isr J Med Sci. 1980 Jan;16(1):33–36. [PubMed] [Google Scholar]
- Handzel Z. T., Levin S., Dolphin Z., Schlesinger M., Hahn T., Altman Y., Schechter B., Shneyour A., Trainin N. Immune competence of newborn lymphocytes. Pediatrics. 1980 Mar;65(3):491–496. [PubMed] [Google Scholar]
- Herberman R. B., Djeu J. Y., Ortaldo J. R., Holden H. T., West W. H., Bonnard G. D. Role of interferon in augmentation of natural and antibody-dependent cell-mediated cytotoxicity. Cancer Treat Rep. 1978 Nov;62(11):1893–1896. [PubMed] [Google Scholar]


