Abstract
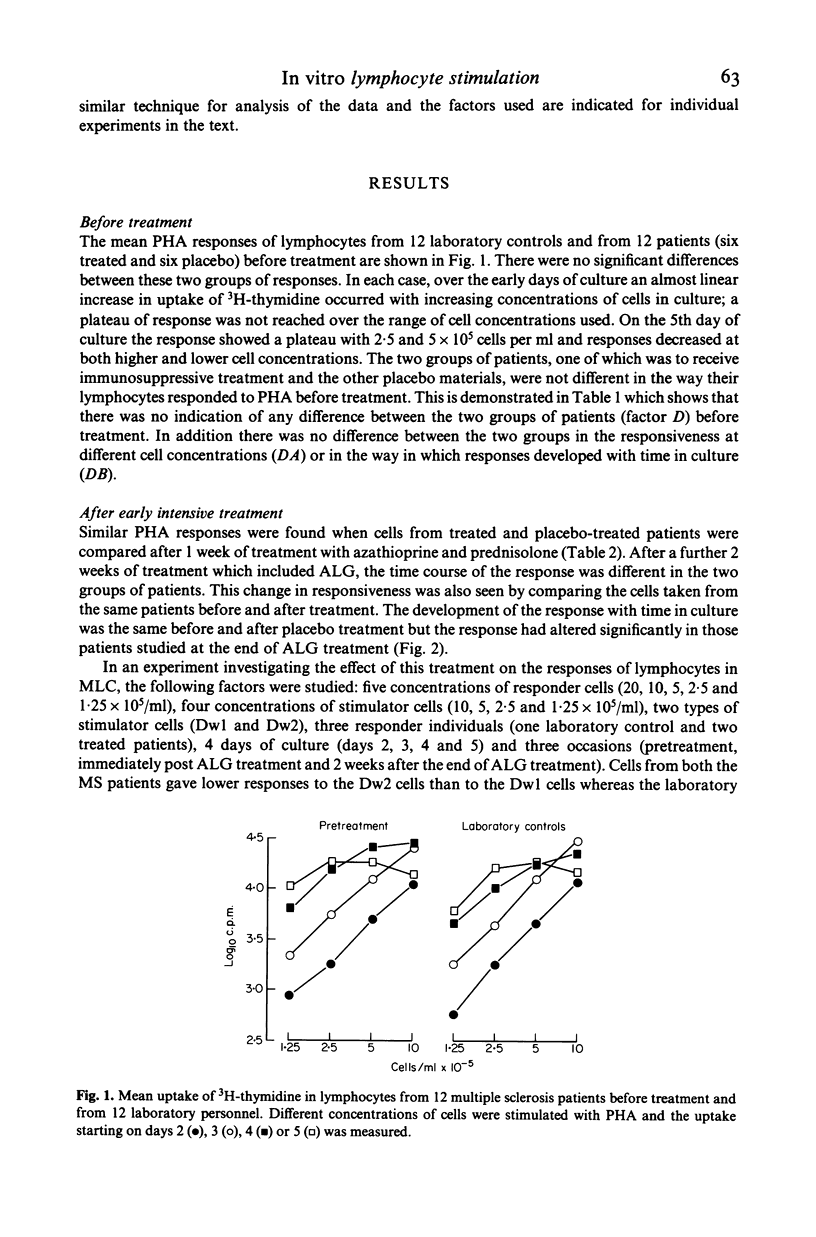
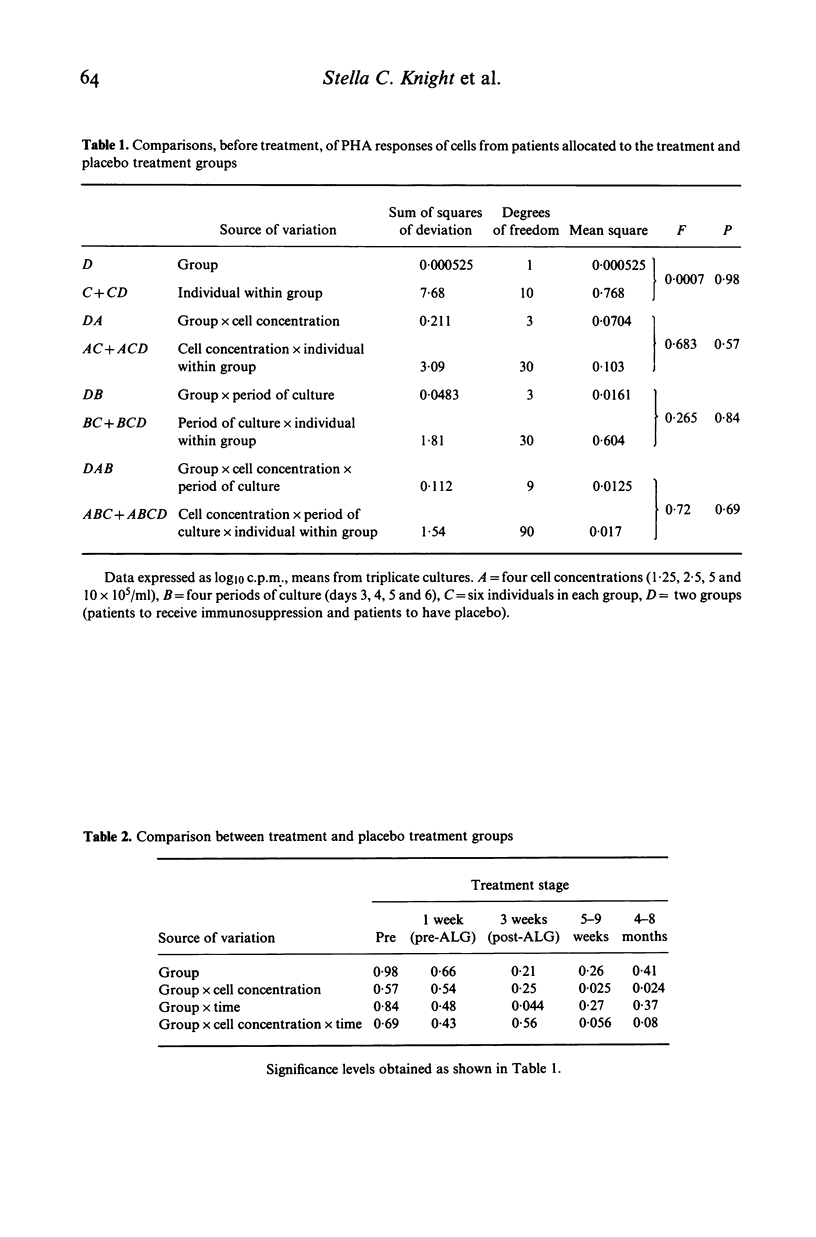
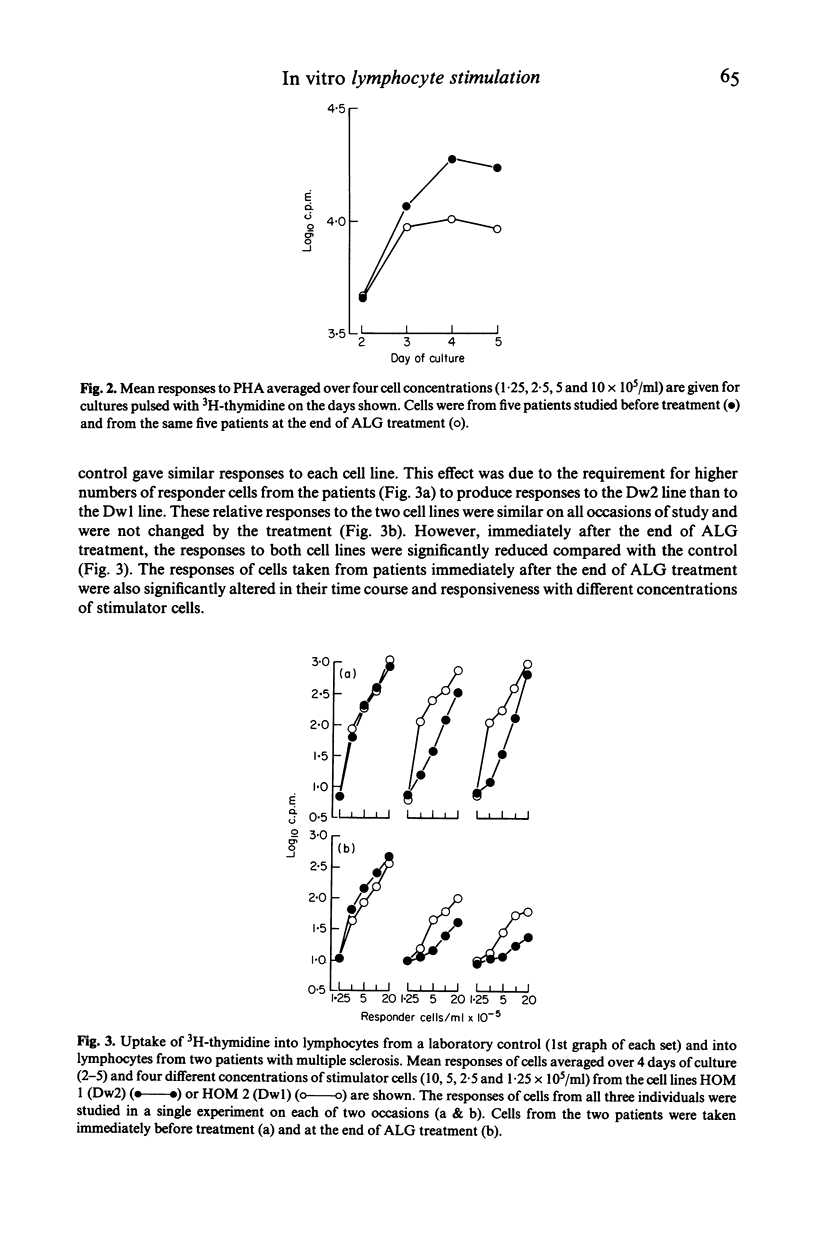
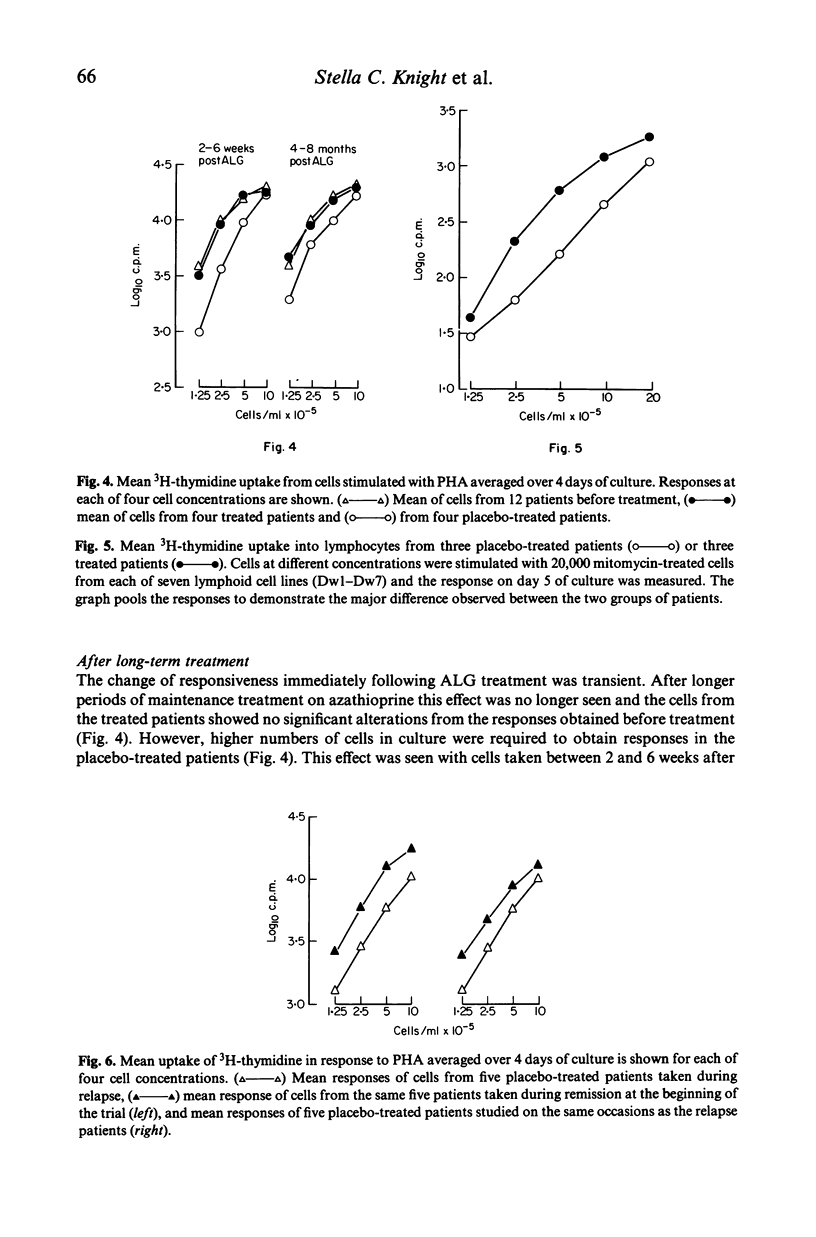
Peripheral blood lymphocytes from 20 patients with clinically definite, relapsing and remitting multiple sclerosis (MS) were studied during their participation in a double-blind trial of immunosuppressive treatment. Proliferative responses occurring with different numbers of cells in culture and on different days of culture in the presence of phytohaemagglutinin (PHA) or with allogeneic cells from lymphoid cell lines (MLC) were assessed. Cells taken from patients before treatment showed similar responses to cells from laboratory personnel. However, when cells were taken from patients in relapse or from untreated patients as the disease progressed, there was an alteration in the pattern of response; higher number of cells were required in culture to produce responses. A change in the responsiveness to PHA or in MLC may therefore accompany the progression of the disease in MS (reflecting clinical relapses and possibly subclinical activity of the disease), perhaps resulting from a simple reduction in the proportion of cells able to respond. After intense immunosuppression followed by long-term maintenance on azathioprine, cells from patients gave similar responses to those found before treatment. Thus long-term immunosuppression prevented the progressive alteration in lymphocyte function. Shifts in the total cell number and time in culture required to allow proliferation with mitogens of cells from untreated MS patients could explain both the 'low' of PHA responses reported and the changes of in vitro 'suppressor' function of these cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnason B. G., Antel J. Suppressor cell function in multiple sclerosis. Ann Immunol (Paris) 1978 Feb-Mar;129(2-3):159–170. [PubMed] [Google Scholar]
- Bach M. A., Phan-Dinh-Tuy F., Tournier E., Chatenoud L., Bach J. F., Martin C., Degos J. D. Deficit of suppressor T cells in active multiple sclerosis. Lancet. 1980 Dec 6;2(8206):1221–1223. doi: 10.1016/s0140-6736(80)92480-0. [DOI] [PubMed] [Google Scholar]
- Basten A., McLeod J. G., Pollard J. D., Walsh J. C., Stewart G. J., Garrick R., Frith J. A., Van Der Brink C. M. Transfer factor in treatment of multiple sclerosis. Lancet. 1980 Nov 1;2(8201):931–934. doi: 10.1016/s0140-6736(80)92100-5. [DOI] [PubMed] [Google Scholar]
- Batchelor J. R. Histocompatibility antigens and their relevance to multiple sclerosis. Br Med Bull. 1977 Jan;33(1):72–77. doi: 10.1093/oxfordjournals.bmb.a071400. [DOI] [PubMed] [Google Scholar]
- Corley R. B. Responses of alloantigen-primed lymphocytes in vitro. Quantitative analysis of the relative frequency of reactive lymphocytes in primed populations which respond to allogeneic stimulating cells. Eur J Immunol. 1977 Feb;7(2):93–99. doi: 10.1002/eji.1830070208. [DOI] [PubMed] [Google Scholar]
- Farrant J., Knight S. C. Help and suppression by lymphoid cells as a function of cellular concentration. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3507–3510. doi: 10.1073/pnas.76.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Newton C. Relative ability to provide help: an explanation for Con A-induced suppression. Clin Exp Immunol. 1981 Sep;45(3):504–513. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. L., Dau P. C., Spitler L. E. Altered regulation of mitogen responsiveness by suppressor cells in multiple sclerosis. Clin Exp Immunol. 1979 Apr;36(1):78–84. [PMC free article] [PubMed] [Google Scholar]
- Hommers O. R., Lamers K. J., Reekers P. Effect of intensive immunosuppression on the course of chronic progressive multiple sclerosis. J Neurol. 1980;223(3):177–190. doi: 10.1007/BF00313182. [DOI] [PubMed] [Google Scholar]
- Huddlestone J. R., Oldstone M. B. T suppressor (TG) lymphocytes fluctuate in parallel with changes in the clinical course of patients with multiple sclerosis. J Immunol. 1979 Oct;123(4):1615–1618. [PubMed] [Google Scholar]
- Ilonen J., Reunanen M., Salmi A., Tiilikainen A. Lymphocyte blast transformation responses and viral antibodies in relation to HLA antigens in multiple sclerosis. J Neurol Sci. 1981 Jan;49(1):117–133. doi: 10.1016/0022-510x(81)90194-5. [DOI] [PubMed] [Google Scholar]
- Knight S. C. Cellular immunity in multiple sclerosis. Br Med Bull. 1977 Jan;33(1):45–50. doi: 10.1093/oxfordjournals.bmb.a071395. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Lance E. M., Abbosh J., Munro A., O'Brien J. Intensive immunosuppression in patients with disseminated sclerosis. III. Lymphocyte response in vitro. Clin Exp Immunol. 1975 Jul;21(1):23–31. [PMC free article] [PubMed] [Google Scholar]
- Mertin J., Knight S. C., Rudge P., Thompson E. J., Healy M. J. Double-blind, controlled trial of immunosuppression in treatment of multiple sclerosis. Lancet. 1980 Nov 1;2(8201):949–951. doi: 10.1016/s0140-6736(80)92107-8. [DOI] [PubMed] [Google Scholar]
- O'Brien J., Knight S., Quick N. A., Moore E. H., Platt A. S. A simple technique for harvesting lymphocytes cultured in Terasaki plates. J Immunol Methods. 1979;27(3):219–223. doi: 10.1016/0022-1759(79)90219-9. [DOI] [PubMed] [Google Scholar]
- Patzold U., Pocklington P. Azathioprine in multiple sclerosis--a 3 year controlled study of its effectiveness. J Neurol. 1980;223(2):97–117. doi: 10.1007/BF00313173. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Weiner H. L., Hauser S. L., Cohen J. A., Distaso J. A., Schlossman S. F. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980 Jul 17;303(3):125–129. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]


