Abstract
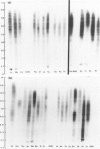
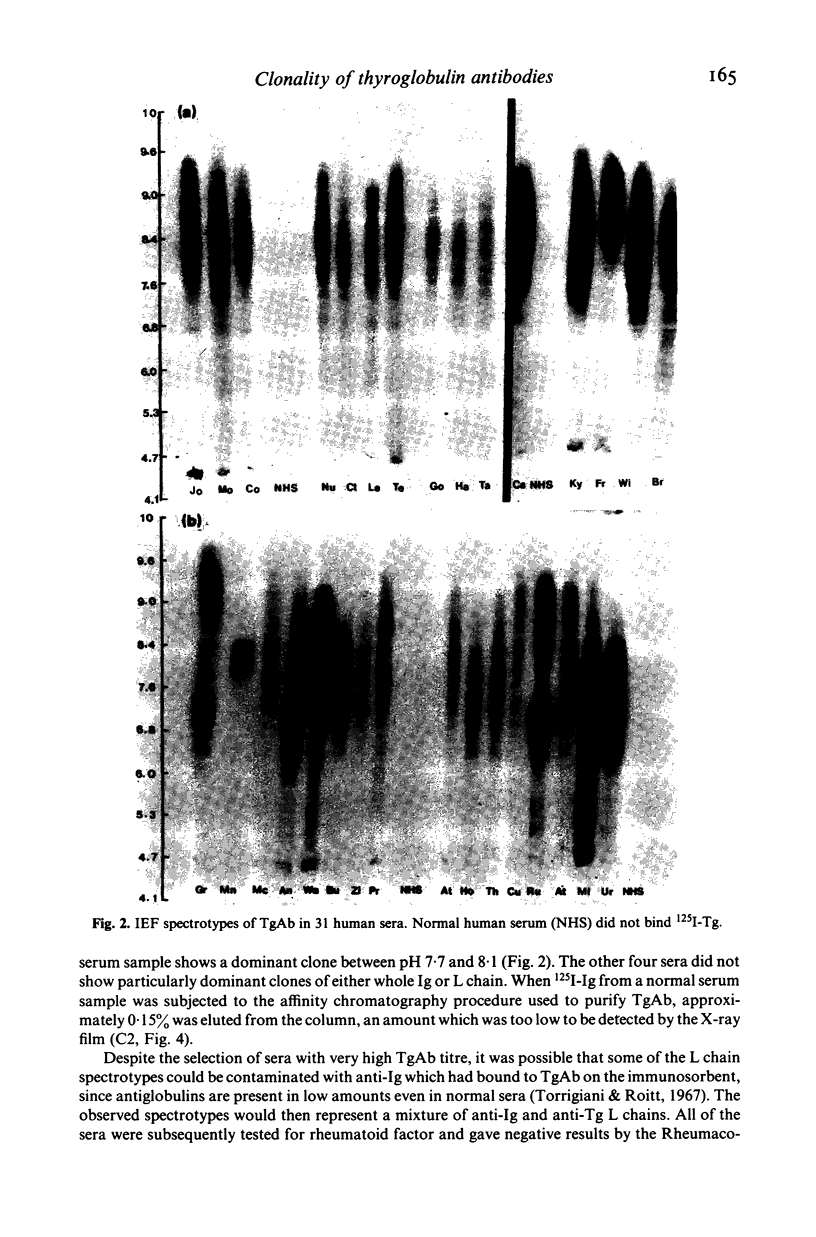
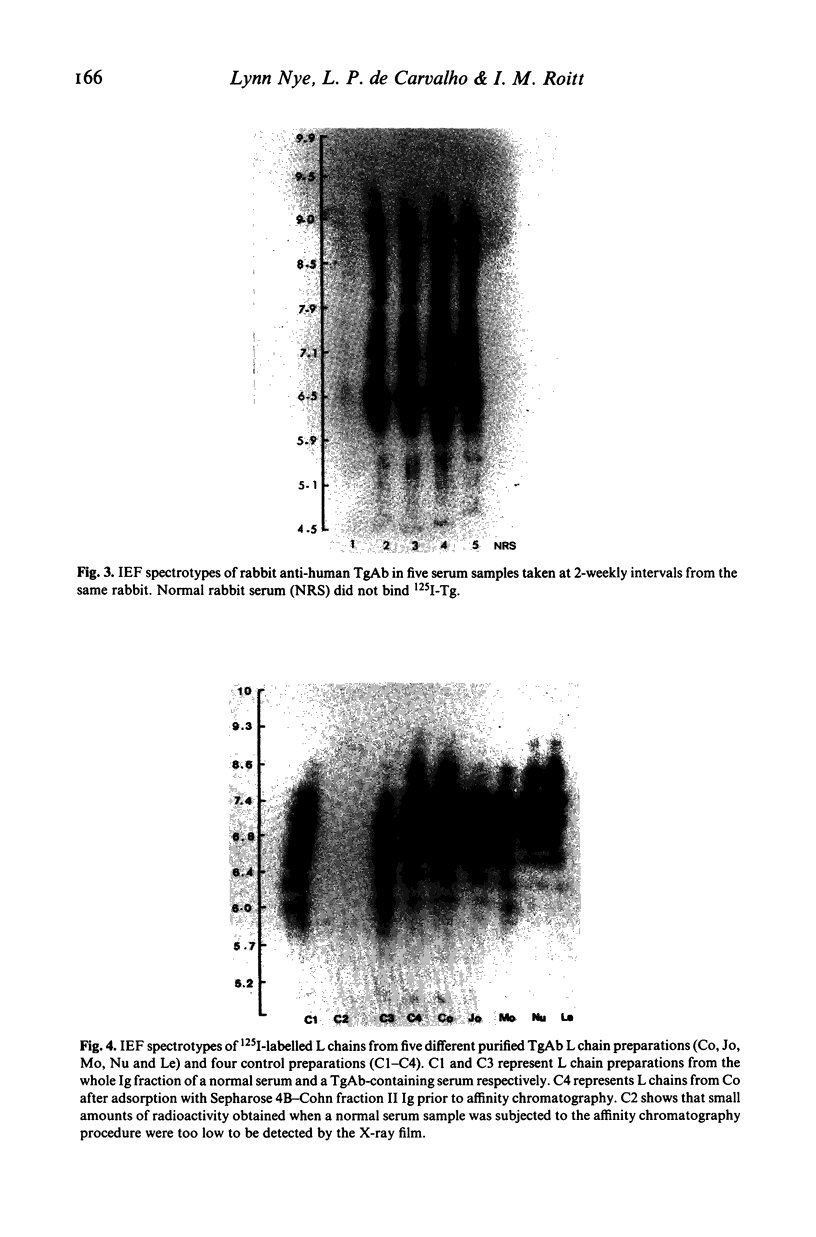
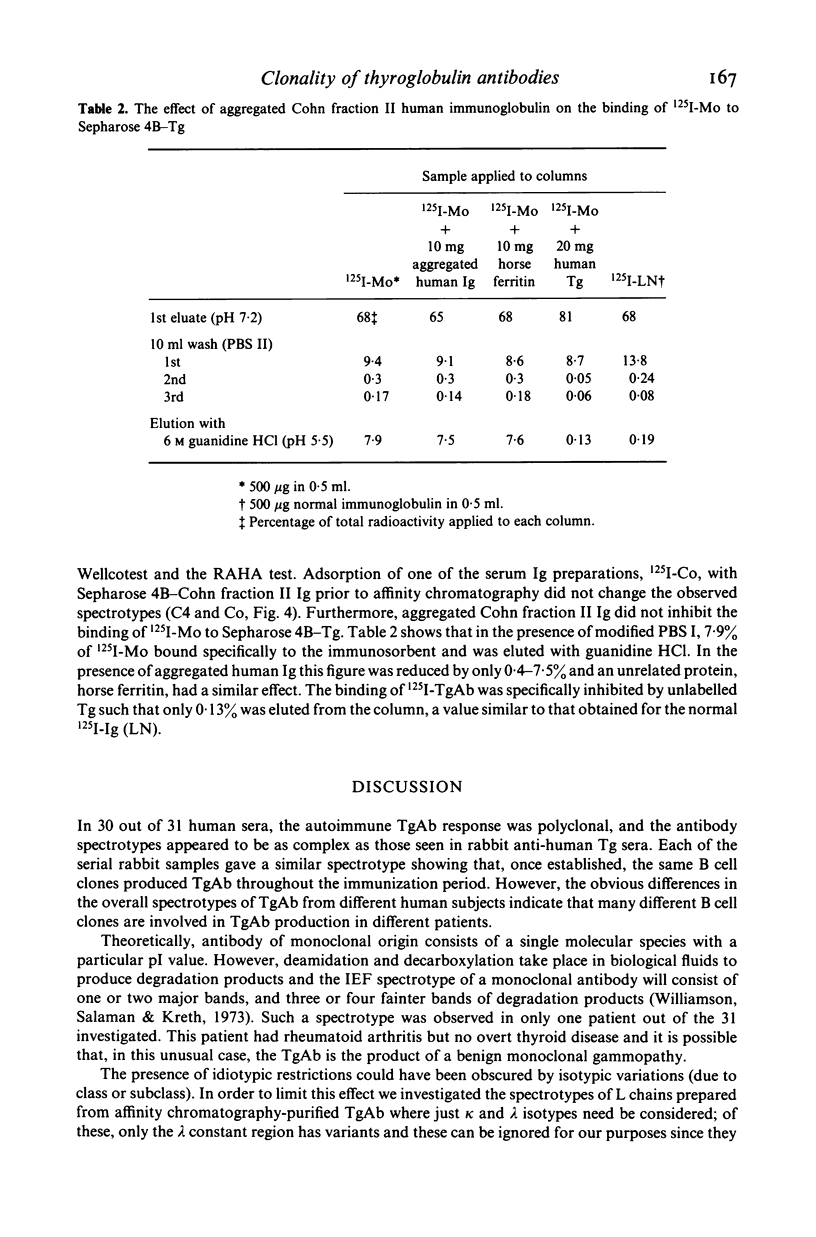
Thyroglobulin antibodies in the sera from 31 patients with a variety of disorders were studied by isoelectric focusing. Only one gave a spectrotype indicative of a monoclonal response, the other 30 giving spectrotypes characteristic of polyclonal responses. There was evidence of clonal dominance in some of the sera and each gave a different spectrotype. Light chains were prepared from five thyroglobulin antibodies purified by affinity chromatography. There was no restriction in the spectrotypes when compared with light chains prepared from normal immunoglobulin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Welch E., Trieshmann H. W., Jr Human triclonal anti-IgG gammopathy. II. Determination of the antigenic specificity patterns of the IgG, IgA and IgM autoantibodies for the subclasses of IgG. Immunology. 1978 Sep;35(3):437–445. [PMC free article] [PubMed] [Google Scholar]
- Adorini L., Harvey M. A., Miller A., Sercarz E. E. Fine specificity of regulatory T cells. II. Suppressor and helper T cells are induced by different regions of hen egg-white lysozyme in a genetically nonresponder mouse strain. J Exp Med. 1979 Aug 1;150(2):293–306. doi: 10.1084/jem.150.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bankhurst A. D., Torrigiani G., Allison A. C. Lymphocytes binding human thyroglobulin in healthy people and its relevance to tolerance for autoantigens. Lancet. 1973 Feb 3;1(7797):226–230. doi: 10.1016/s0140-6736(73)90066-4. [DOI] [PubMed] [Google Scholar]
- Eichmann K. Expression and function of idiotypes of lymphocytes. Adv Immunol. 1978;26:195–254. doi: 10.1016/s0065-2776(08)60231-x. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Lackland H., Hood L., Krause R. M. Induction of rabbit antibody with molecular uniformity after immunization with group C streptococci. J Exp Med. 1970 Jan 1;131(1):207–221. doi: 10.1084/jem.131.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., GOODMAN H. ANTIBODY ACTIVITY IN SIX CLASSES OF HUMAN IMMUNOGLOBULINS. Science. 1964 Feb 7;143(3606):588–590. doi: 10.1126/science.143.3606.588. [DOI] [PubMed] [Google Scholar]
- Forre O., Natvig J. B., Michaelsen T. E. Cross-idiotypic reactions among anti-Rh (D) antibodies. Scand J Immunol. 1977;6(10):997–1003. doi: 10.1111/j.1365-3083.1977.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Gibson D. M. Species specificity in the isoelectric spectra of immunoglobulin light chains. J Immunol. 1977 Feb;118(2):409–411. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hay F. C., Torrigiani G. The distribution of anti-thyroglobulin antibodies in the immunoglobulin G subclasses. Clin Exp Immunol. 1973 Dec;15(4):517–521. [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kunkel H. G., Agnello V., Joslin F. G., Winchester R. J., Capra J. D. Cross-idiotypic specificity among monoclonal IgM proteins with anti- -globulin activity. J Exp Med. 1973 Feb 1;137(2):331–342. doi: 10.1084/jem.137.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
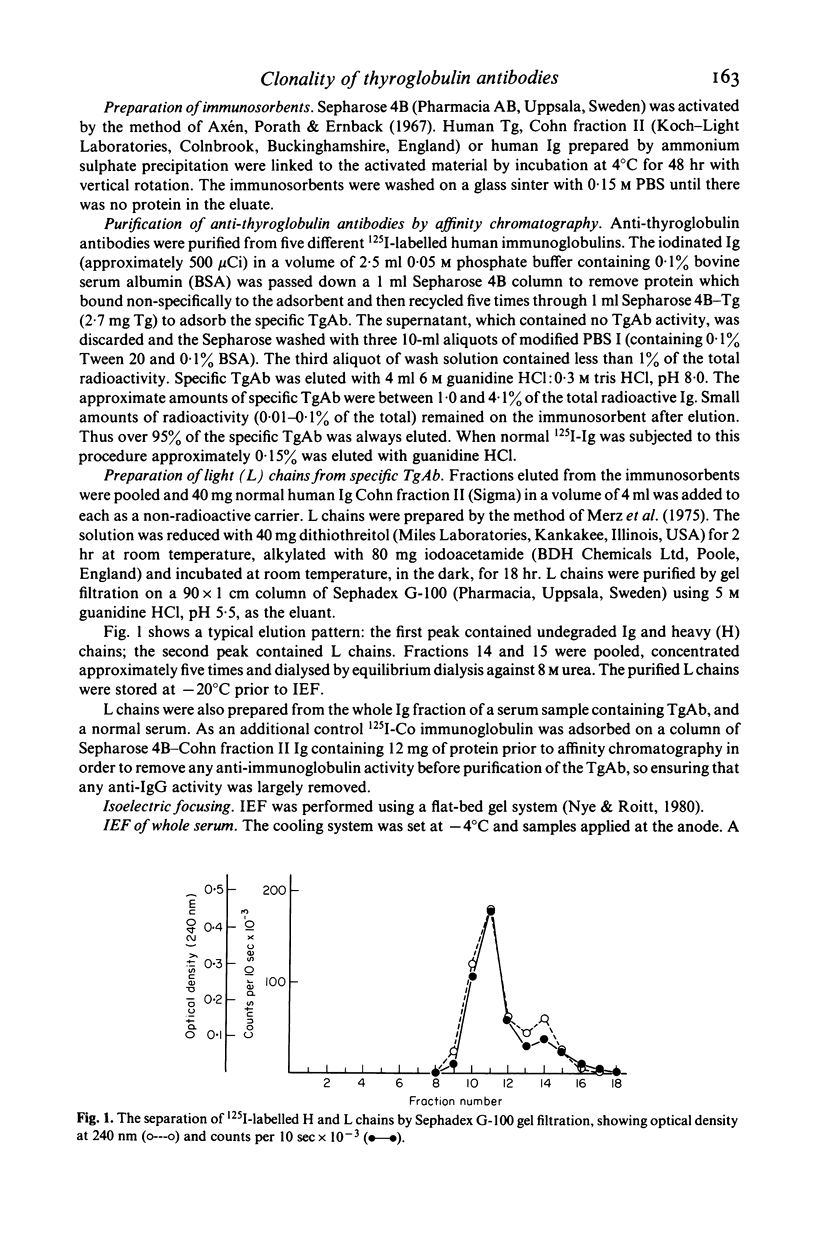
- Merz D. C., Finstad C. L., Litman G. W., Good R. A. Aspects of vertebrate immunoglobulin evolution. Constancy in light chain electrophoretic behavior. Immunochemistry. 1975 Jul;12(6-7):499–504. doi: 10.1016/0019-2791(75)90074-9. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Pincus J. H. Molecular restriction of anti-DNP antibodies induced by dinitrophenylated type 3 pneumococcus. J Immunol. 1973 Jul;111(1):42–51. [PubMed] [Google Scholar]
- Montgomery P. C., Rockey J. H., Kahn R. L., Skandera C. A. Molecular restriction of anti-DNP antibodies induced by (DNP)2-gramicidin S. J Immunol. 1975 Oct;115(4):904–910. [PubMed] [Google Scholar]
- Nye L., Pontes de Carvalho L. C., Roitt I. M. Restrictions in the response to autologous thyroglobulin in the human. Clin Exp Immunol. 1980 Aug;41(2):252–263. [PMC free article] [PubMed] [Google Scholar]
- Nye L., Roitt I. M. Isoelectric focusing of human antibodies directed against a high molecular weight antigen. J Immunol Methods. 1980;35(1-2):97–103. doi: 10.1016/0022-1759(80)90154-4. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Briles D. E., Davie J. M. Complete sharing of light chain spectrotypes by murine IgM and IgG anti-streptococcal antibodies. J Immunol. 1977 Jun;118(6):2161–2166. [PubMed] [Google Scholar]
- ROITT I. M., CAMPBELL P. N., DONIACH D. The nature of the thyroid auto-antibodies present in patients with Hashimoto's thyroiditis (lymphadenoid goitre). Biochem J. 1958 Jun;69(2):248–256. doi: 10.1042/bj0690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE N. R., WITEBSKY E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956 Jun;76(6):417–427. [PubMed] [Google Scholar]
- Richards F. F., Pincus J. H., Bloch K. J., Barnes W. T., Haber E. The relationship between antigenic complexity and heterogeneity in the antibody response. Biochemistry. 1969 Apr;8(4):1377–1384. doi: 10.1021/bi00832a011. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Salsano F., Froland S. S., Natvig J. B., Michaelsen T. E. Same idiotype of B-lymphocyte membrane IgD and IgM. Formal evidence for monoclonality of chronic lymphocytic leukemia cells. Scand J Immunol. 1974;3(6):841–846. doi: 10.1111/j.1365-3083.1974.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Saito T., Tada T. Antigen-specific suppressive factor produced by a transplantable I-J bearing T-cell hybridoma. Nature. 1979 Apr 5;278(5704):555–558. doi: 10.1038/278555a0. [DOI] [PubMed] [Google Scholar]
- Torrigiani G., Roitt I. M. Antiglobulin factors in sera from patients with rheumatoid arthritis and normal subjects. Quantitative estimation in different immunoglobulin classes. Ann Rheum Dis. 1967 Jul;26(4):334–340. doi: 10.1136/ard.26.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieshmann H. W., Jr, Abraham G. N., Santucci E. A. The characterization of human anti-IgG autoantibodies by liquid isoelectric focussing. J Immunol. 1975 Jan;114(1 Pt 1):176–181. [PubMed] [Google Scholar]
- WEIGLE W. O. The immune response of rabbits tolerant to bovine serum albumin to the injection of other heterologous serum albumins. J Exp Med. 1961 Jul 1;114:111–125. doi: 10.1084/jem.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. R., Salaman M. R., Kreth H. W. Microheterogeneity and allomorphism of proteins. Ann N Y Acad Sci. 1973 Jun 15;209:210–224. doi: 10.1111/j.1749-6632.1973.tb47530.x. [DOI] [PubMed] [Google Scholar]
- Willims R. C., Jr, Kunkel H. G., Capra J. D. Antigenic specificities related to the cold agglutinin activity of gamma M globulins. Science. 1968 Jul 26;161(3839):379–381. doi: 10.1126/science.161.3839.379. [DOI] [PubMed] [Google Scholar]