Abstract
Therapeutic vaccination with Copaxone (glatiramer acetate, Cop-1) protects motor neurons against acute and chronic degenerative conditions. In acute degeneration after facial nerve axotomy, the number of surviving motor neurons was almost two times higher in Cop-1-vaccinated mice than in nonvaccinated mice, or in mice injected with PBS emulsified in complete Freund's adjuvant (P < 0.05). In mice that express the mutant human gene Cu/Zn superoxide dismutase G93A (SOD1), and therefore simulate the chronic human motor neuron disease amyotrophic lateral sclerosis, Cop-1 vaccination prolonged life span compared to untreated matched controls, from 211 ± 7 days (n = 15) to 263 ± 8 days (n = 14; P < 0.0001). Our studies show that vaccination significantly improved motor activity. In line with the experimentally based concept of protective autoimmunity, these findings suggest that Cop-1 vaccination boosts the local immune response needed to combat destructive self-compounds associated with motor neuron death. Its differential action in CNS autoimmune diseases and neurodegenerative disorders, depending on the regimen used, allows its use as a therapy for either condition. Daily administration of Cop-1 is an approved treatment for multiple sclerosis. The protocol for non-autoimmune neurodegenerative diseases such as amyotrophic lateral sclerosis, remains to be established by future studies.
Amyotrophic lateral sclerosis (ALS) is a progressive disease of the upper and lower motor neurons, in most cases causing death by respiratory failure. Its etiology, prognosis, and progression have been intensively studied over the last decade. Two etiological factors have so far been identified: mutations in the Cu/Zn superoxide dismutase (SOD) gene on chromosome 21 (1) and in the alsin gene putatively encoding a ras GTPase (2, 3). These mutations, however, account for fewer than 10% of patients with ALS. Many factors that contribute to the pathogenesis of ALS are common to other chronic degenerative disorders of the central nervous system (CNS), such as oxidative stress, excitotoxicity, deprivation of trophic support, and ionic imbalance (4). The pathogenesis of chronic selective death of anterior horn cells can be studied in transgenic mice expressing the mutant human Cu/Zn SOD G93A (SOD1) gene (5, 6).
There have been numerous clinical and experimental attempts to halt the progression of ALS by blocking different mediators of cytotoxicity (7). Because not all ALS patients have defective genes, the results of such attempts are often verified by studying motor neuron death (common to all cases of ALS) in an animal model of acute peripheral nerve axotomy (8, 9). The only drug currently used to slow down the progression of ALS, although with only modest effect, is riluzole, a putative blocker of glutamate release (10, 11).
The immune system, which protects the organism from the effects of invasion by pathogenic microorganisms, was recently found to be protective against destructive self-components as well (12–16). In acute neurodegenerative conditions caused by mechanical (e.g., crush injury or axotomy) (12, 14) or biochemical insults (e.g., glutamate or oxidative stress) (15), more neurons survive in the presence of an evoked anti-self T cell-mediated response than in its absence, provided that the evoked response is well regulated (13, 16–19). The protective T cell-mediated response can be boosted, without risk of antoimmune disease induction, by administration of copolymer-1 (Cop-1; Copaxone), a synthetic polypeptide consisting of the amino acids tyrosine, glutamate, alanine, and lysine (15, 20). It was recently suggested that this compound can activate a wide range of self-reactive T cells (20, 21). In a model of a chronic neurodegenerative disorder associated with optic nerve neuropathy, such as glaucoma, Cop-1 vaccination was found to bypass the tissue-specificity barrier imposed by antigens residing in the damaged tissue (15, 22) and to significantly increase neuronal survival (15, 20).
Cop-1 is a Food and Drug Administration-approved drug for the treatment of multiple sclerosis (MS). In this study, by treating mice with Cop-1 (according to a different regimen from that used for MS), we show that motor neurons can be protected against both acute and chronic degeneration.
Materials and Methods
Acute Motor Neuron Disorder.
Adult female mice (12 weeks old, 20–25 g) of the C57BL/6JO1aHsd strain (Harlan Winkelmann, Borchen, Germany) were subjected to unilateral facial nerve axotomy. Mice in the experimental group received a total of 100 μg of Cop-1 in complete Freund's adjuvant (CFA). Control animals were axotomized and were either untreated or injected with PBS emulsified in CFA. Seven days later a facial–facial anastamosis was created in anesthetized mice (100 mg of Ketaset plus 5 mg of Rompun per kg of body weight) by microsurgical reconnection of the proximal stump to the distal stump with two 11-0 epineural sutures (Ethicon EH 7438G, Norderstedt, Germany). The wound was closed with three 4-0 skin sutures. For assessment of recovery, facial motor neurons supplying the whiskerpad muscles were retrogradely labeled by injection of 30 μl of 1% aqueous solution of the fluorescent retrograde tracer FluoroGold plus 2% dimethyl sulfoxide (DMSO) injected into the muscles of each whisker pad. Seven days later, the mice were re-anesthetized and perfused transcardially with 0.9% NaCl followed by fixation with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 20 min. The brains were removed and 50-μm-thick coronal sections were cut through the brain stems with a vibratome. Sections were observed with a Zeiss Axioskop 50 epifluorescence microscope through a custom-made HQ-Schmalband-filter set for FluoroGold (AHF Analysentechnik, Tubingen, Germany).
Quantitative Analysis.
For image analysis, a charged coupled device video camera system (Optronics Engineering Model DEI-470, Goleta, CA) combined with the image analyzing software Optimas 6.5 (Optimas, Bothell, WA) was used to manually count the retrogradely labeled facial motor neurons on the computer screen (23). Employing the fractionator principle (24), all retrogradely labeled motor neurons with visible cell nuclei were counted in every second section of the 50-μm-thick sections through the facial nucleus on both the operated and the unoperated side. Counting was done by two observers who were blinded to the treatment received by the rats.
Electrophysiological Assessment.
The two large hairs of the C-row on each side of the face were used for biometric analysis. With the mice under light ether narcosis, all other vibrissae were clipped with small fine scissors. A digital camcorder (Panasonic NV DX-110 EG) was used to videotape the actively exploring mice for 3–5 min. After calibration, video images of whisking behavior were sampled at 50 Hz (50 fields per sec), with the video camera shutter opened for 4 msec. Images were recorded on AY-DVM 60 EK minicassettes. The video sequences were slowly reviewed and 1.5-sec sequence fragments from each mouse were selected for analysis of whisking biometrics. The selection criteria used were stable position of the head, frequency of whisking, and degree of vibrissal protraction. The selected sequences were captured by a 2D/Manual Advanced Video System PEAK Motus 2000 (PEAK Performance Technologies, Englewood, CO). The spatial model consisted of three reference points (tip of the nose and the inner angles of both eyes). Each vibrissa is represented in the spatial model by two points: its base and a point on the shaft 0.5 cm from the base.
Glutamate Injection.
With the aid of a binocular microscope, the right eye of the anesthetized mouse was punctured in the upper part of the sclera with a 27-gauge needle, and a 10-μl Hamilton syringe with a 30-gauge needle was inserted as far as the vitreal body. Mice were injected with l-glutamate (200 nmoles) (Sigma) dissolved in saline (total volume 1 μl).
Labeling of Retinal Ganglion Cells.
Mice were anaesthetized as described above and placed in a stereotactic device. The skull was exposed, and the bregma was identified and marked. The site selected for injection was in the superior colliculus, 2.92 mm posterior to the bregma, 0.5 mm lateral to the midline, and at a depth of 2 mm from the brain surface. A window was drilled in the scalp above the designated coordinates in the right and left hemispheres. The neurotracer dye FluoroGold (5% solution in saline, Fluorochrome, Denver) was stereotactically applied (1 μl, at a rate of 0.5 μl/min in each hemisphere) by using a Hamilton syringe, and the skin over the wound was sutured.
Assessment of Retinal Ganglion Cell (RGC) Survival.
At the end of the experimental period, the mice were killed by injection of a lethal dose of pentobarbitone (170 mg/kg). Their eyes were enucleated and the retinas were detached and prepared as flattened whole mounts in 4% paraformaldehyde in PBS. Labeled cells from four to six fields of identical size (0.076 mm2) were counted. The counted fields were located at approximately the same distance from the optic disk (0.3 mm) to allow for variations in RGC density as a function of distance from the optic disk. Fields were counted under the fluorescence microscope (magnification, ×800) by observers blinded to the treatment received by the mice. The average number of RGCs per field was calculated for each retina. The number of RGCs in the contralateral (uninjured) eye was also counted, and served as an internal control.
ALS Model.
Transgenic mice [B6SJL−TgN (SOD1−G93A) 1Gur, supplied by The Jackson Laboratory], aged 60 days, were vaccinated with Cop-1 (75 μg) emulsified in complete Freund's adjuvant (CFA; Difco) containing 5 mg/ml Mycobacterium tuberculosis. The emulsion (total volume 200 μl) was injected into the hind foot pad, and thereafter the mice were treated daily with oral Cop-1 (12.5 mg/kg/day) given in the drinking water. Their motor activity and mortality were monitored. The transgene in these mice carries a mutant human SOD1 allele containing the Gly-93 → Ala (G93A) gene. Paralysis is caused by the progressive loss of motor neurons from the spinal cord. Control mice were either left untreated or received 30 mg/kg riluzole daily. A second group of transgenic mice, expressing more copies of the defective SOD1 mutants, was treated by vaccination with Cop-1 mixed with Alum-Phos. This was administered either as two injections given a week apart or as three injections. The first injection was given when the mice were ≈60–70 days old, the second injection was given a week later, and the third injection was given a month later. Control groups received either a single injection or two injections of Alum-Phos.
The mice were allowed to grasp and hold onto a vertical wire (2 mm diameter) with a small loop at the lower end. Their activity was recorded individually by a computerized system and assessed daily. For statistical evaluation, the rotarod activity was normalized to the mean activity of each mouse from day 40 to day 60.
Results
Cop-1 Vaccination Protects Against Motor Neuron Death Induced by Acute Facial Nerve Axotomy.
Transection of the facial nerve in the adult mouse is known to cause an easily visible late degeneration of 20–35% of the axotomized motor neurons (25). Mice were immunized with Cop-1 (n = 10) or injected with PBS (n = 9), both emulsified in CFA, and 7 days later were subjected to facial nerve axotomy. Mice in a third group (n = 8) were axotomized without prior immunization, and mice in a fourth group (n = 7) were left intact. Eight weeks after axotomy, as shown in Fig. 1 and Table 1, the mean number of FluoroGold-labeled motor neurons in the mice vaccinated with Cop-1 was significantly larger than the number obtained in the group injected with PBS in CFA or in the untreated control group (P < 0.05). Immunization with PBS in CFA had no protective effect. Treatment with Cop-1 had no effect on the number of motor neurons in the unlesioned facial nucleus.
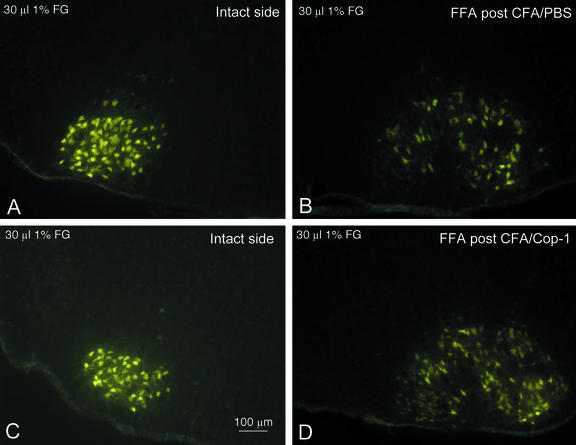
Figure 1.
Rescue of motor neurons by Cop-1 administered after facial nerve axotomy. Retrograde neuronal labeling after injection of FluoroGold into the whiskerpad showed no differences in the localization or amount of motor neurons in the intact facial nucleus between mice immunized with PBS in CFA (A) and mice injected with Cop-1 in CFA (C). In contrast, the lesioned facial nucleus in control mice pretreated with PBS in CFA contained significantly fewer labeled motor neurons than that of the lesioned facial nucleus in mice pretreated with Cop-1 in CFA (B vs. D).
Table 1.
Effect of Cop-1 vaccination on survival of motor neurons
| Group | Unlesioned facial nucleus | Lesioned facial nucleus |
|---|---|---|
| A: Intact mice (n = 7) | 1,559 ± 135 | 1,707 ± 90*B,C,D |
| B: FFA only (n = 8) | 1,434 ± 106 | 670 ± 178*A,D |
| C: FFA after PBS/CFA injection (n = 9) | 1,605 ± 142 | 766 ± 104*A,D |
| D: FFA after vaccination with Cop-1 in CFA (n = 10) | 1,640 ± 186 | 1,172 ± 152*A,B,C |
Numerical values of the results shown in Fig. 1. Data are presented as means ± SD. Differences between the experimental groups were detected by applying a one-way analysis of variance (ANOVA) and a post hoc t test for unpaired data with Bonferroni–Holm's correction.
Cop-1 Administration Preserves Motor Neuron Activity After Acute Axotomy.
To determine whether the larger number of motor neurons found in the Cop-1-treated axotomized mice than in the controls was associated with functional improvement, we biometrically analyzed whisking behavior. Baseline parameters of whisking behavior were documented in intact control mice. Under normal physiological conditions, the mystacial vibrissae are erect with anterior orientation. Their simultaneous sweeps, known as “whisking” or “sniffing” (26, 27), occur 5–11 times per second (28, 29). The key movements of this motor activity are the protraction and retraction of the vibrissal hairs by the piloerector muscles, which are innervated by the buccal branch of the facial nerve (30). When the facial nerve is transected, the vibrissae acquire a caudal orientation and remain motionless. We used this model (Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org) to evaluate the following parameters: (i) protraction (forward movement of the vibrissae), measured by the rostrally opened angle between the mid-sagittal plane and the hair shaft (large protractions are represented by small angle values); (ii) whisking frequency, represented by cycles of protraction and retraction (passive backward movement) per second; (iii) amplitude (the difference, in degrees, between maximal retraction and maximal protraction; (iv) angular velocity during protraction (in degrees per second); and (v) angular acceleration during protraction (in degrees per second per second).
Mice subjected to facial nerve axotomy and Cop-1 administration exhibited significantly better whisking activity than the other groups of mice, as demonstrated by the amplitude, the angular velocity during protraction, and the angular acceleration during protraction (Table 2).
Table 2.
Effect of Cop-1 vaccination on recovery of whisking behavior after facial nerve axotomy
| Group | Frequency, Hz | Angle at maximal protraction, ° | Amplitude, ° | Angular velocity during protraction, °/sec | Angular acceleration during protraction, °/sec2 |
|---|---|---|---|---|---|
| A: Intact mice (n = 7) | 6.0 ± 1.0 | 65.1 ± 22 | 40 ± 14*B,C | 627 ± 346*B,C,D | 20,084 ± 1508*B,C,D |
| B: FFA only (n = 8) | 5.0 ± 2.0 | 81.2 ± 27 | 11.0 ± 6.0*A,D | 75 ± 43*A | 1,655 ± 1146*A |
| C: FFA treated with PBS/CFA (n = 9) | 5.3 ± 1.2 | 64.4 ± 6.3 | 22.1 ± 9.9*A,D | 214 ± 70*A | 3,874 ± 889*A |
| D: FFA treated with Cop-1/CFA (n = 10) | 5.5 ± 0.9 | 68.2 ± 23.05 | 38.9 ± 10.6*B,C | 347.8 ± 87.3*A | 6,713 ± 2071*A |
Biometrics of normal and recovering whisking behavior in intact mice (group A) and in mice subjected to FFA only (group B), mice subjected to FFA after injection of PBS in CFA (group C), and mice subjected to FFA after injection of Cop-1 in CFA (group D). Values are means ± SD. Superscript letters indicate groups with significantly different values (*, P < 0.05).
The results presented above suggest that motor neurons in a mouse model of an acute degenerative disorder can benefit from protection induced by Cop-1 vaccination.
Cop-1 Treatment Increases the Life Expectancy of ALS Mice.
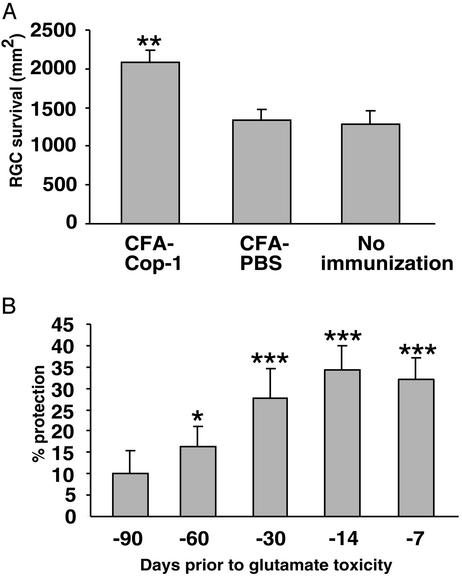
Mice overexpressing the defective human SOD1 gene develop a motor disease that closely resembles the human disease ALS. The motor dysfunction eventually causes their death. To assess the potential efficacy of Cop-1 vaccination in a model of a chronic neurodegenerative disorder, we first determined whether the effect of a single immunization with Cop-1 emulsified in CFA is long-lived. The selected dose was 75 μg, because this was found to be optimally effective in a range of tested Cop-1 dosages between 25 and 225 μg (H.S., Hila Avidan, and M.S., unpublished data). We used a mouse model of glutamate toxicity to first measure the length of time that a single vaccination with Cop-1 emulsified in CFA protects against glutamate toxicity. Mice were subjected to local toxicity of glutamate, which was injected intravitreally at different time intervals after vaccination. One week after the glutamate injection, the number of surviving neurons were counted. Fig. 2A shows that a single injection with Cop-1 emulsified in CFA was significantly more effective in protecting against glutamate toxicity than the injection of PBS emulsified in CFA. Significant protection against glutamate toxicity was observed in mice that had received toxic amounts of glutamate up to 60 days (but not more) after the vaccination (Fig. 2B). The weak effect of the adjuvant by itself (Figs. 1 and 2A), coupled with the persistent nature of postvaccination immune-dependent protection, encouraged us to examine the efficacy of Cop-1 immunization for chronic ALS.
Figure 2.
Cop-1 vaccination protects RGCs against glutamate toxicity. (A) C57BL/6J mice (n = 6) were immunized with Cop-1 emulsified in CFA. Ten days later the mice were subjected to unilateral intraocular injection of toxic amounts of glutamate (200 nmol). As controls, we used a group of mice injected with glutamate only (n = 7) and a group of mice (n = 8) that were immunized with PBS in CFA 10 days before being exposed to glutamate. Three days after their exposure to glutamate, the RGCs were retrogradely labeled. Retinas were excised 1 week after their exposure to glutamate. RGC survival was assessed by counting the labeled cells, and is expressed as mean ± SEM per mm2. A two-tailed Student's t test was used for statistical analysis. (B) C57BL/6J mice were immunized with Cop-1 in CFA at the indicated time points before receiving unilateral intraocular injections of toxic amounts of glutamate (200 nmol). After an additonal 7 days they were killed, their retinas were excised, and RGC survival was assessed. Mice that received only glutamate were used as controls. Protection is expressed in terms of RGC survival, calculated as a percentage of RGC survival in control mice.
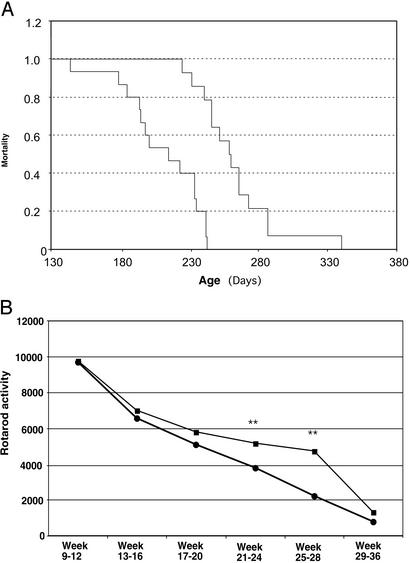
Mice immunized with Cop-1 at the age of 60 days and age-matched untreated control mice were observed daily and weighed weekly. The mice received a single immunization of Cop-1 emulsified in CFA, followed by oral immunization with Cop-1 given in the drinking water. The age at symptom onset was determined as the age (in days) at the time of first appearance of tremors or shaking of the limbs, or hanging (rather than splaying out) of the hind limbs when the mouse was held in the air by the tail. Loss of the righting reflex was taken to indicate the end stage of the disease. In the absence of treatment, the mice in our study (n = 14) died at the age of 211 ± 7 days (mean ± SD). Mice immunized with Cop-1 (n = 15) lived for 263 ± 8 days. Thus, vaccination with Cop-1 dramatically increased the life expectancy of the ALS mice (Fig. 3A). As a positive control, 15 mice were given a daily dose (30 mg/kg) of riluzole, the only drug currently given to ALS patients. Those mice survived for 230 ± 7 days. In addition to the increase of almost 25% in life span, disease onset (manifested by motor performance) was delayed (Fig. 3B), indicating that the benefit was also expressed in the quality of life, both at preclinical and at clinical stages (Fig. 3B). Normal values for each mouse were obtained by assessing nocturnal motor activity (from 8 p.m. to 8 a.m.) between the ages of 40 and 60 days, by using the rotarod apparatus (Laser- und Medizin-Technologie, Berlin). With the object of translating these findings into a future treatment modality for ALS patients, we tested G93A transgenic mice, which express a large number of copies of the human mutant form of SOD1, using Cop-1 emulsified in CFA or in Alum–Phos (as CFA is not suitable for human therapy), or Cop-1 without adjuvant. The optimal protocol has not yet been found. The increase in lifespan did not exceed 10% in the high-copy model (data not shown). Further studies are needed to establish the optimal dosage and regimen needed for this purpose.
Figure 3.
Life expectancy in ALS mice immunized with Cop-1. (A) Nonvaccinated controls (n = 15) became paralyzed in one or more limbs and died by the age of 211 ± 7 days (mean ± SEM). Cop-1-treated mice survived for 263 ± 8 days. Survival data (expressed by mortality as a function of age in days) were analyzed by the Mantel–Cox test or Cox's proportional hazards regression analysis. Statistical significance was tested by one-way ANOVA, followed by a post hoc Student–Neuman–Keuls procedure with the SPSS-PC software program (SPSS, Chicago). (B) Average rotary activity measured at the indicated time points in Cop-1-treated and untreated mice. Data are expressed as the mean ± SEM. Rotarod testing and weight were compared by ANOVA. Statistical significance was tested by one-way ANOVA followed by a post hoc Student–Neuman–Keuls procedure with the SPSS-PC software program. Differences between treated and untreated mice were observed between days 12 and 20 (P < 0.06), between weeks 21 and 24 (P < 0.008), and between weeks 25 and 28 (P < 0.002).
Discussion
The results show that vaccination with Cop-1, in a protocol different from that used for MS patients, protects motor neurons from acute or chronic degeneration in a mouse model.
It was recently suggested that CNS insult acts as a distress signal to the immune system (13–16). Relative to normal rodents, those deprived of mature T cells lose significantly more neurons after a CNS insult (13, 15). Experimental evidence suggests that, under stress, the CNS signals to the immune system, evoking an adaptive immune response that is directed against abundant antigens residing at the site of the lesion (15, 16, 31, 32). Individuals differ in their ability to spontaneously evoke such an immune response (13). However, all individuals can benefit from boosting of the response, provided that intervention occurs at the right time, and uses the right antigen in optimal formulation so that the anti-self response is intensified, yet does not increase the risk of autoimmune disease (12, 17, 33). We further demonstrated that the same T cells (T helper 1) could apparently be responsible both for autoimmune disease and for protection from the detrimental effects of destructive self-compounds. Thus, T helper 1 cells directed against immunodominant proteins were shown to be capable of inducing autoimmune disease, as well as neuroprotection (34). Disease-free protection was achieved by inducing an immune response against cryptic epitopes residing within the same potentially pathogenic immune-abundant protein, or by using an altered pathogenic peptide to eliminate the potential pathogenicity of the peptide (17, 32).
Studies aimed at uncovering the mechanism behind this protection have revealed that T cells directed against the self-antigen migrate toward the lesion site where they become activated. Once activated, they can serve as a source of cytokines and neurotrophins. In addition, while the local neural cells clear the site of injury of cell debris and other deleterious matter (35, 36), the T cells can regulate the local innate response in which resident cells become either antigen-presenting cells or phagocytic, buffering cells (I. Shaked, O. Butovsky, T. Mizrahi, R. Gersner, X. Xiao, P. Soteropoulos, P. Tolias, R. P. Hart, and M.S., unpublished data; and ref. 37).
In an attempt to develop a method for boosting this T cell-dependent response while circumventing tissue barrier specificity and genetic susceptibility, we tested the copolymer Cop-1, an approved drug for MS. Cop-1 vaccination has been shown to protect CNS neurons against death induced by optic nerve injury or by glutamate toxicity in rat and mouse models (15, 38). In these experiments, as well as in others where vaccination was used to induce neuroprotection, we used animals immunized with PBS in CFA as controls. The protection provided by CFA was rarely significant relative to untreated controls, and was always significantly less than the optimal protection obtained by immunization with the specific antigens. In the present work too, mice treated with PBS in CFA and with PBS alone were included as control groups in the acute model of motor neuron degeneration. As expected, CFA was found to have some protective effect; however, it was significantly lower than that obtained in mice treated with Cop-1 in CFA. Recent studies have shown that Cop-1 immunization without adjuvant also leads to effective protection against glutamate toxicity (H.S., Hila Avidan, and M.S., unpublished data). This immunization method provides protection comparable to that achieved in immunization with adjuvant, but the level of T cells that can sustain the effect is retained for only two weeks. Moreover, repeated daily vaccinations with Cop-1 not only do not improve the outcome, but they even diminish the benefit derived from a single injection (H.S., Hila Avidan, and M.S., unpublished data). It seems reasonable to assume that Cop-1 immunization leading to neuroprotection, like protective immunization with self-derived peptides, is phenotype dependent. Therefore, repeated immunization on a daily basis, even if it leads to the presence of a large number of reactive T cells, does not lead to neuroprotection if the phenotype is shifted. Recent studies showed that for the immunization with Cop-1 to be protective, IFN-γ expression by the activated lymphocytes must be sustained. This might explain why an immunization protocol that is effective in suppressing autoimmunity in an autoimmune disease such as MS is not beneficial against neurodegenerative, such as ALS, disorders where active immunity is needed (20). Thus, it appears that to develop Cop-1 for use as a therapeutic vaccine against chronic disease, it is necessary to find the formulation and the frequency of immunization. Future studies should focus on the optimal timing, frequency, and dosage of the intervention, and other adjuvants should be tested as well. In current studies, the use of Cop-1 without any vehicle is under investigation. An important finding of the present study is that the choice of animal model with regard to the level of expression of the mutant form of the human SOD1 gene might significantly influence the success of the immune intervention and perhaps also affect the protocol.
ALS is an aggressive neurodegenerative disorder in which many destructive self-components, not all of them identified, play a major role. Among the principal mediators of toxicity identified to date are glutamate and oxidative stress. The role of cellular and molecular immune factors in protecting the organism against the effects of these self-destructive agents has been debated over the years. Researchers and clinicians have also attempted to use immunosuppressants as a treatment, on the assumption that in ALS, as in many other neurodegenerative diseases, inflammation may be associated with disease propagation and therefore deleterious (39, 40). Also, the presence of anti-ganglioside antibodies in ALS patients (41) has led some researchers to suggest that ALS is an autoimmune disease. However, there is no conclusive evidence for any of these hypotheses, and therapy with immunosuppressants, including whole-body irradiation, has failed to show any effect (42). This failure might suggest that the observed autoimmunity in ALS does not contribute to the ongoing degeneration, and that the autoimmunity associated with the disease might in part reflect a failure to recruit an appropriate protective immune mechanism to cope with the threat to the tissue. Accordingly, by boosting a well controlled immunity that simulates or cross-reacts with weak self-reacting T cells, it is possible to counteract the destructive effect of self-compounds such as glutamate (15, 19, 43). Recent evidence suggests that elements of neurodegenerative and autoimmune disorders can be intermingled in the same disease Processes known to occur in degenerative diseases have been detected in autoimmune diseases (20, 44–48). It is thus possible that in “mixed” disorders the degenerative tissue will benefit from immunomodulation rather than from immunosuppression (43). Thus, unlike repeated injections as in the therapeutic protocol for MS, a one-time vaccination with Cop-1 can be viewed as a therapy in which immune activity is stimulated and modulated rather than suppressed.
The antibiotic minocycline was recently shown to delay the onset and slow the progression of symptoms in a mouse model of ALS (49–51). In view of the fact that this drug, like other tetracyclines, has various anti-inflammatory actions, its observed beneficial effect in ALS mice might appear to contradict the finding of the present study. It appears, however, that minocycline in the ALS model works by blocking the release of cytochrome c in the mitochondria (50). Thus, the two treatment modalities are not contradictory, and might even be complementary, a possibility that is worth investigating.
The pathogenesis of ALS is thought to be related to an insufficiency of glutamate transporters, whose function is to buffer an excess of extracellular glutamate (52, 53). IFN-γ up-regulates the expression of glutamate transporters by astrocytes (54). Because IFN-γ is a dominant cytokine in T helper 1 cells, our group has suggested that the activity of T cell-derived IFN-γ might underlie a mechanism whereby the T cells, once they home to the lesion site, are activated, and assist the resident microglia in their task of clearing away cell debris and other toxic substances that threaten the tissue. Studies by our group have indeed demonstrated that the phagocytic activity of microglia and their capacity for uptake of radioactive glutamate are significantly increased after their exposure to IFN-γ (I. Shaked, O. Butovsky, T. Mizrahi, R. Gersner, X. Xiao, P. Soteropoulos, P. Tolias, R. P. Hart, and M.S., unpublished data).
Other studies by our group have shown that Cop-1-reactive T cells, when injected into uninjured rats, home to the CNS, a feature characteristic of T cells that recognize self-antigens (38). The number of Cop-1-reactive T cells that home to the CNS is increased after CNS injury (55). It therefore seems likely that Cop-1-reactive T cells recognize self-antigens presented at the site of the lesion. However, because Cop-1 is not identical in structure to any self-reacting antigen, the T cells it activates are probably those that respond with low affinity, i.e., not the potentially pathogenic ones. It was suggested that Cop-1 acts like an altered peptide ligand in activating nonencephalitogenic T cells (21). The use of Cop-1, which was characterized as a weak self-reacting antigen that cross-reacts with a wide range of self-reacting T cells (20, 21), might be a way to satisfy the diverse requirements among human individuals with respect to safe self-reactive antigens. In a model of glaucoma, a disease characterized by chronic optic nerve neuropathy, Cop-1 vaccination was found to induce a transient increased infiltration of T cells, and proved itself to be a powerful tool for tissue protection. This was seen when retinas of animals immunized with Cop-1 showed a better morphology than retinas of noninjured animals, both subjected to glutamate toxicity (15). It is therefore conceivable that, as in other CNS insult models, in the present model vaccination transiently increases the number of infiltrating T cells (37), thereby boosting the ability of resident cells to mediate tissue maintenance and repair.
ALS associated with SOD mutations represents only a small fraction of ALS patients. Therefore, the promising results with Cop-1 obtained here in the acute peripheral nerve injury model in addition to its effect in the transgenic mice argue in favor of Cop-1 as a treatment for other forms of ALS besides to the familial disease. On the assumption that the number of causative or risk factors in ALS or any other motor neuron disease is large, it is unlikely that global protection can be achieved by a single drug targeted against a single mediator of toxicity. Global protection, providing the multiple factors needed for CNS recovery, might be obtained by a therapeutic strategy in which the body's protective (immune system) resources are harnessed. Because T cells are prominent among the immune participants in neuroprotection (13, 16), such therapy would presumably recruit a myriad of T cell-derived factors (35, 36).
In view of the present findings, and because Cop-1 has been approved by the Food and Drug Administration for clinical use in MS, we suggest that it should immediately be developed (in a clinically approved formulation and regimen) into a vaccine for the treatment of peripheral nerve injury and motor neuron diseases.
Supplementary Material
Abbreviations
- ALS
amyotrophic lateral sclerosis
- SOD
superoxide dismutase
- MS
multiple sclerosis
- RGC
retinal ganglion cell
- CFA
complete Freund's adjuvant
References
- 1.Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan J P, Deng H X, et al. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Hadano S, Yanagisawa Y, Skaug J, Fichter K, Nasir J, Martindale D, Koop B F, Scherer S W, Nicholson D W, Rouleau G A, et al. Genomics. 2001;71:200–213. doi: 10.1006/geno.2000.6392. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Hentati A, Deng H X, Dabbagh O, Sasaki T, Hirano M, Hung W Y, Ouahchi K, Yan J, Azim A C, et al. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 4.Ludolph A C, Munch C. Drug Metab Rev. 1999;31:619–634. doi: 10.1081/dmr-100101938. [DOI] [PubMed] [Google Scholar]
- 5.Gurney M E. J Neurol Sci. 1997;152,(Suppl. 1):S67–S73. doi: 10.1016/s0022-510x(97)00247-5. [DOI] [PubMed] [Google Scholar]
- 6.Subramaniam J R, Lyons W E, Liu J, Bartnikas T B, Rothstein J, Price D L, Cleveland D W, Gitlin J D, Wong P C. Nat Neurosci. 2002;5:301–307. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- 7.Ludolph A C, Meyer T, Riepe M W. J Neurol. 2000;247:I7–16. doi: 10.1007/s004150050552. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Martin L J. J Histochem Cytochem. 2001;49:957–972. doi: 10.1177/002215540104900804. [DOI] [PubMed] [Google Scholar]
- 9.Martin L J, Price A C, Kaiser A, Shaikh A Y, Liu Z. Int J Mol Med. 2000;5:3–13. doi: 10.3892/ijmm.5.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Doble A, Kennel P. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:301–312. doi: 10.1080/146608200300079545. [DOI] [PubMed] [Google Scholar]
- 11.Meininger V, Lacomblez L, Salachas F. J Neurol. 2000;247:19–22. [PubMed] [Google Scholar]
- 12.Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, et al. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen I R, Schwartz M. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 15.Schori H, Kipnis J, Yoles E, WoldeMussie E, Ruiz G, Wheeler L A, Schwartz M. Proc Natl Acad Sci USA. 2001;98:3398–3403. doi: 10.1073/pnas.041609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen I R, Weiner H, Schwartz M. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauben E, Agranov E, Gothilf A, Nevo U, Cohen A, Smirnov I, Steinman L, Schwartz M. J Clin Invest. 2001;108:591–599. doi: 10.1172/JCI12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauben E, Mizrahi T, Agranov E, Schwartz M. Eur J Neurosci. 2002;16:1731–1740. doi: 10.1046/j.1460-9568.2002.02241.x. [DOI] [PubMed] [Google Scholar]
- 19.Schori H, Yoles E, Wheeler L A, Schwartz M. Eur J Neurosci. 2002;16:557–564. doi: 10.1046/j.1460-9568.2002.02134.x. [DOI] [PubMed] [Google Scholar]
- 20.Kipnis J, Schwartz M. Trends Mol Med. 2002;8:319–323. doi: 10.1016/s1471-4914(02)02373-0. [DOI] [PubMed] [Google Scholar]
- 21.Hafler D A. J Clin Invest. 2002;109:581–584. doi: 10.1172/JCI15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakalash S, Kipnis J, Yoles E, Schwartz M. Invest Ophthalmol Vis Sci. 2002;43:2648–2653. [PubMed] [Google Scholar]
- 23.Dohm S, Streppel M, Guntinas-Lichius O, Pesheva P, Probstmeier R, Walther M, Neiss W F, Stennert E, Angelov D N. Restor Neurol Neurosci. 2000;16:117–126. [PubMed] [Google Scholar]
- 24.Gundersen H J. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- 25.Sendtner M, Gotz R, Holtmann B, Escary J L, Masu Y, Carroll P, Wolf E, Brem G, Brulet P, Thoenen H. Curr Biol. 1996;6:686–694. doi: 10.1016/s0960-9822(09)00450-3. [DOI] [PubMed] [Google Scholar]
- 26.Semba K, Szechtman H, Komisaruk B R. Brain Res. 1980;195:281–298. doi: 10.1016/0006-8993(80)90065-7. [DOI] [PubMed] [Google Scholar]
- 27.Welker W I. Behaviour. 1964;22:223–244. [Google Scholar]
- 28.Carvell G E, Simons D J. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komisaruk B R. J Comp Physiol Psychol. 1970;70:482–492. doi: 10.1037/h0028709. [DOI] [PubMed] [Google Scholar]
- 30.Dorfl J. J Anat. 1985;142:173–184. [PMC free article] [PubMed] [Google Scholar]
- 31.Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Proc Natl Acad Sci USA. 2002;99:15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizrahi T, Hauben E, Schwartz M. J Immunology. 2002;169:5971–5977. doi: 10.4049/jimmunol.169.10.5971. [DOI] [PubMed] [Google Scholar]
- 33.Fisher J, Levkovitch-Verbin H, Schori H, Yoles E, Butovsky O, Kaye J F, Ben-Nun A, Schwartz M. J Neurosci. 2001;21:136–142. doi: 10.1523/JNEUROSCI.21-01-00136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kipnis J, Mizrahi T, Yoles E, Ben-Nun A, Schwartz M. J Neuroimmunol. 2002;130:78–85. doi: 10.1016/s0165-5728(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 35.Barouch R, Schwartz M. FASEB J. 2002;16:1304–1306. doi: 10.1096/fj.01-0467fje. [DOI] [PubMed] [Google Scholar]
- 36.Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen I R, Schwartz M. J Autoimmun. 2000;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 37.Butovsky O, Hauben E, Schwartz M. FASEB J. 2001;15:1065–1067. doi: 10.1096/fj.00-0550fje. [DOI] [PubMed] [Google Scholar]
- 38.Kipnis J, Yoles E, Porat Z, Cohen A, Mor F, Sela M, Cohen I R, Schwartz M. Proc Natl Acad Sci USA. 2000;97:7446–7451. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niebroj-Dobosz I, Domitrz I, Mickielewicz A. Folia Neuropathol. 1999;37:107–312. [PubMed] [Google Scholar]
- 40.Appel S H, Simpson E P. Curr Neurol Neurosci Rep. 2001;1:303–305. doi: 10.1007/s11910-001-0081-z. [DOI] [PubMed] [Google Scholar]
- 41.Alaedini A, Latov N. J Immunoassay. 2000;21:377–386. doi: 10.1080/01971520009349543. [DOI] [PubMed] [Google Scholar]
- 42.Drachman D B, Fishman P S, Rothstein J D, Motomura M, Lang B, Vincent A, Mellits E D. Adv Neurol. 1995;68:59–65. [PubMed] [Google Scholar]
- 43.Schwartz M, Kipnis J. Trends Immunol. 2002;23:530–534. doi: 10.1016/s1471-4906(02)02322-0. [DOI] [PubMed] [Google Scholar]
- 44.Bjartmar C, Trapp B D. Curr Opin Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Bjartmar C, Kinkel R P, Kidd G, Rudick R A, Trapp B D. Neurology. 2001;57:1248–1252. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- 46.De Stefano N, Narayanan S, Francis G S, Arnaoutelis R, Tartaglia M C, Antel J P, Matthews P M, Arnold D L. Arch Neurol. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- 47.Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. J Neuroimmunol. 2000;107:161–166. doi: 10.1016/s0165-5728(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 48.Steinman L. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 49.Kriz J, Nguyen M D, Julien J P. Neurobiol Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 50.Van Den Bosch L, Tilkin P, Lemmens G, Robberecht W. NeuroReport. 2002;13:1067–1070. doi: 10.1097/00001756-200206120-00018. [DOI] [PubMed] [Google Scholar]
- 51.Zhu S, Stavrovskaya I G, Drozda M, Kim B Y, Ona V, Li M, Sarang S, Liu A S, Hartley D M, Wu du C, et al. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 52.Fukamachi S, Furuta A, T, I, Ikenoue T, Kaneoka T, Rothstein J D, Iwaki T. Brain Res Dev Brain Res. 2001;132:131–139. doi: 10.1016/s0165-3806(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 53.Howland D S, Liu J, She Y, Goad B, Maragakis N J, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, et al. Proc Natl Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu S, Sheng W S, Ehrlich L C, Peterson P K, Chao C C. Neuroimmunomodulation. 2000;7:153–159. doi: 10.1159/000026433. [DOI] [PubMed] [Google Scholar]
- 55.Moalem G, Monsonego A, Shani Y, Cohen I R, Schwartz M. FASEB J. 1999;13:1207–1217. doi: 10.1096/fasebj.13.10.1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.