Abstract
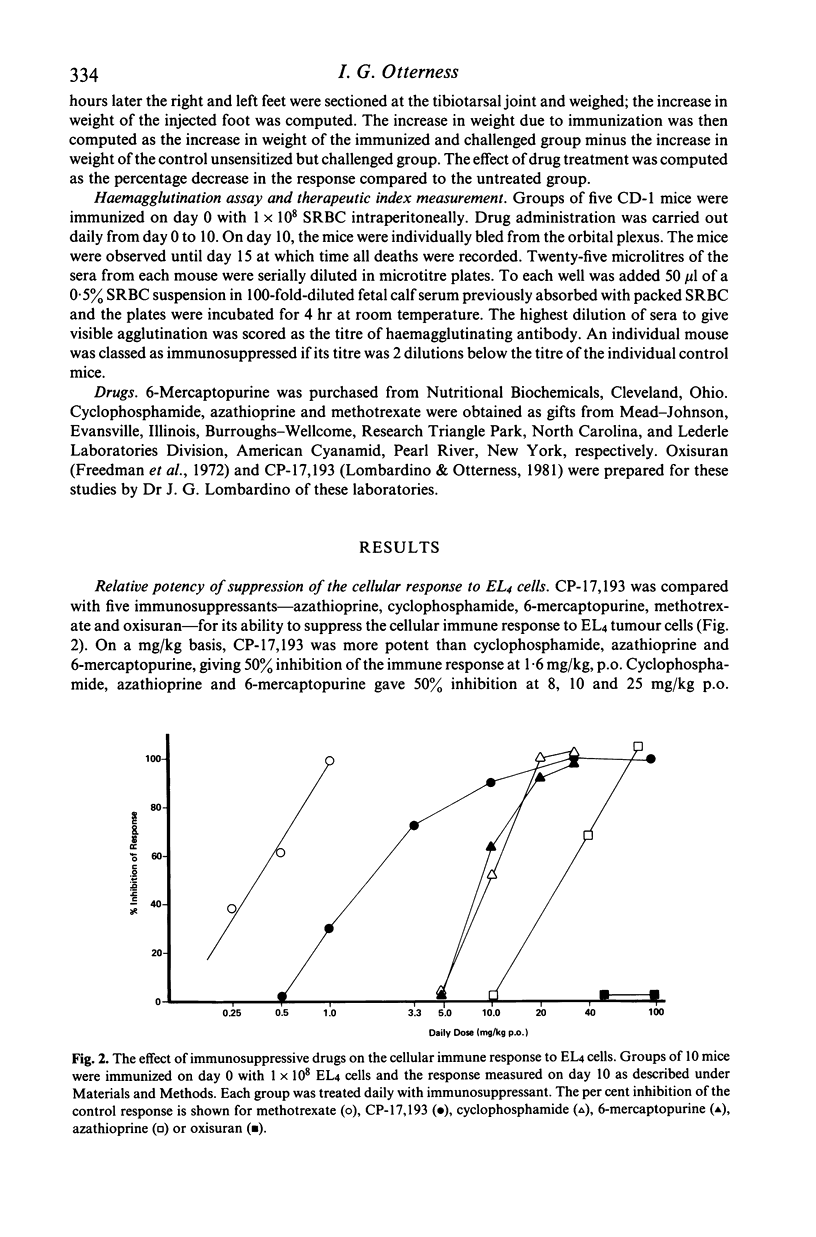
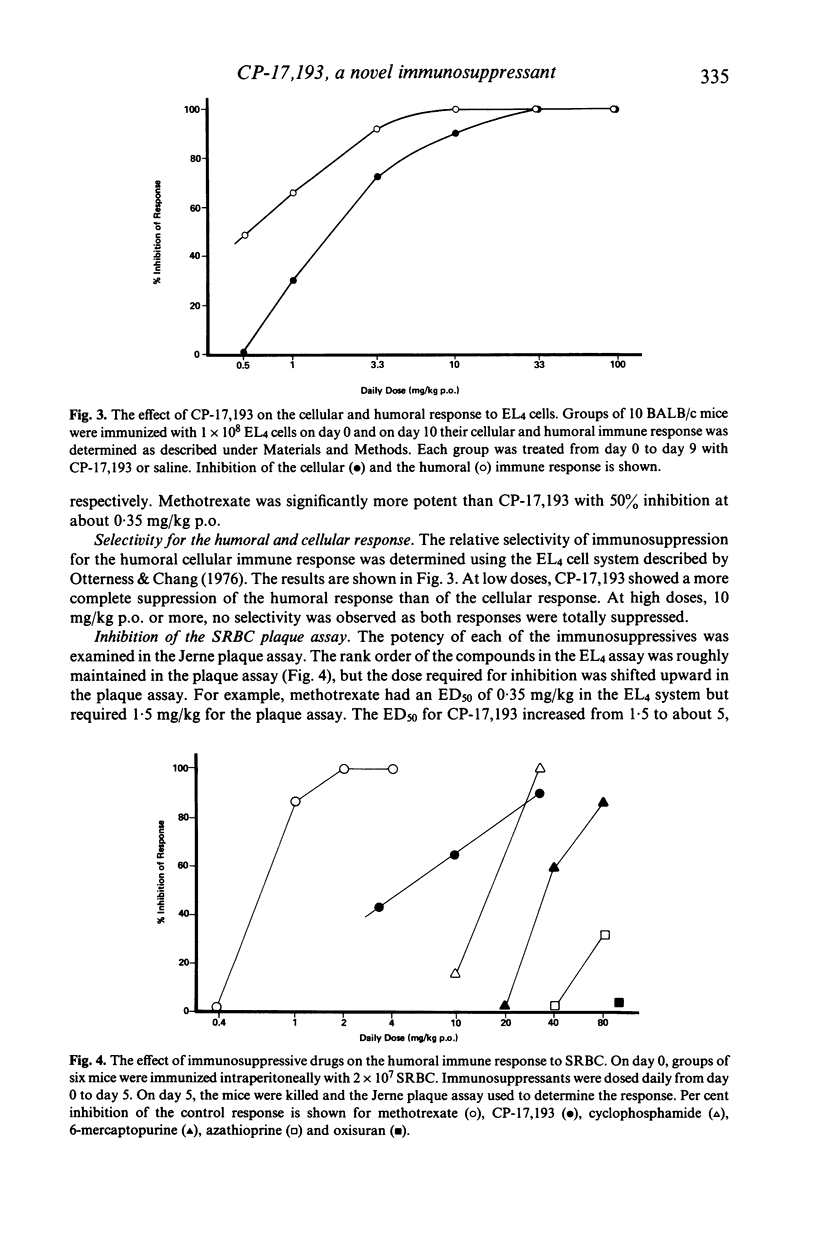
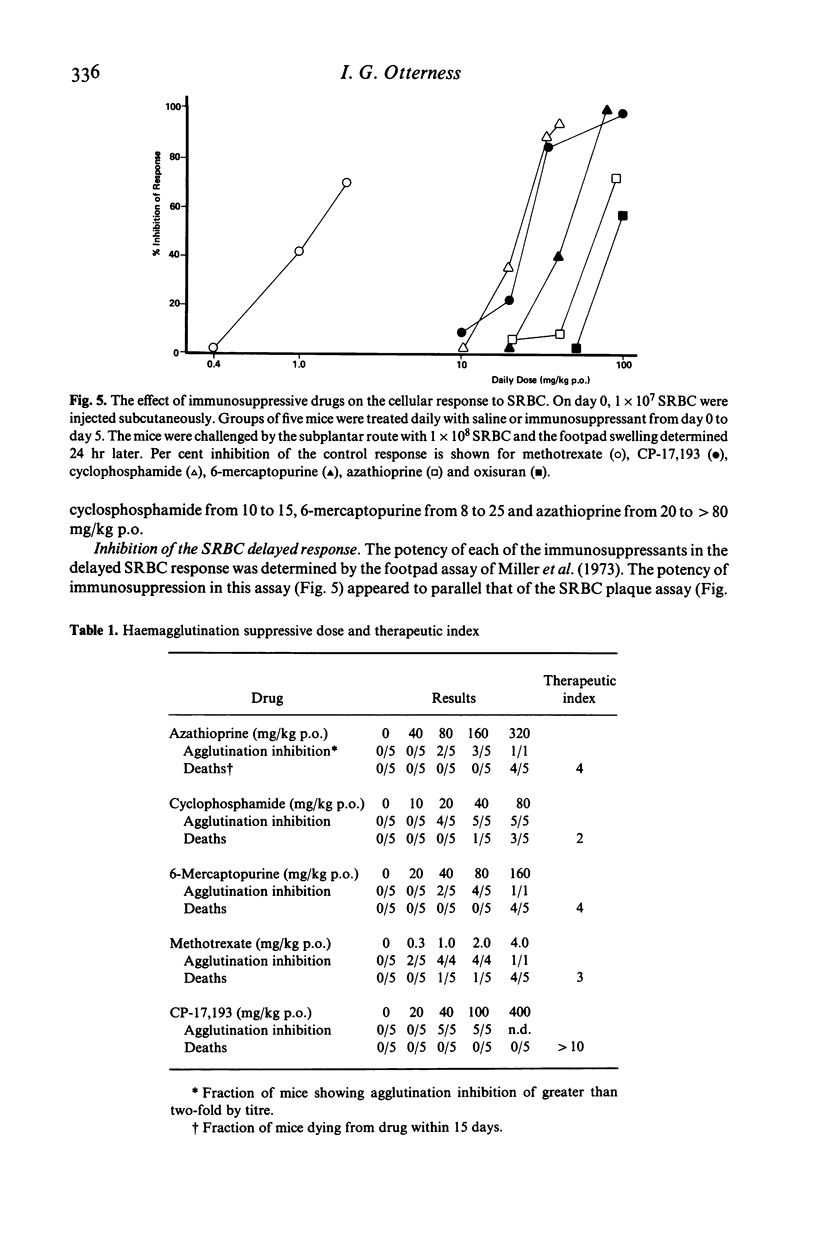
A new immunosuppressant, 2-(4'-chlorophenyl)benzothiopyrano-(4,3-c)pyrazol-3-one, i.e. CP-17,193 was compared to several standard immunosuppressants with respect to its ability to depress immune responses to two antigens, EL4 tumour cells and sheep erythrocytes (SRBC). The order of suppressive potency on a weight basis was methotrexate greater than CP-17,193 greater than cyclophosphamide greater than 6-mercaptopurine greater than azathioprine greater than oxisuran for both antigens. In general, it took more immunosuppressant to inhibit the response to SRBC than to EL4. This resulted in a different therapeutic index for immunosuppression against each antigen. CP-17,193 and cyclophosphamide preferentially inhibited the humoral immune response and were the only agents demonstrating such selectivity. CP-17,193's favourable therapeutic index and its structural dissimilarity from the antimetabolite and alkylating immunosuppressants suggests that it may act through a novel mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERENBAUM M. C., BROWN I. N. DOSE-RESPONSE RELATIONSHIPS FOR AGENTS INHIBITING THE IMMUNE RESPONSE. Immunology. 1964 Jan;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Fox A. E., Gingold J. L., Freedman H. H. Inhibition by oxisuran of cell-mediated hypersensitivity by decrease in numbers of specifically sensitized cells. Infect Immun. 1973 Oct;8(4):549–554. doi: 10.1128/iai.8.4.549-554.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman H. H., Fox A. E., Shavel J., Jr, Morrison G. C. Oxisuran: a differential inhibitor of cell-mediated hypersensitivity. Proc Soc Exp Biol Med. 1972 Mar;139(3):909–912. doi: 10.3181/00379727-139-36264. [DOI] [PubMed] [Google Scholar]
- Huber B., Cantor H., Shen F. W., Boyse E. A. Independent differentiative pathways of Ly1 and Ly23 subclasses of T cells. Experimental production of mice deprived of selected T-cell subclasses. J Exp Med. 1976 Oct 1;144(4):1128–1133. doi: 10.1084/jem.144.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino J. G., Otterness I. G. Novel immunosuppressive agents. Potent immunological activity of some bensothiopyrano [4,3-c]pyrazol-3-ones. J Med Chem. 1981 Jul;24(7):830–834. doi: 10.1021/jm00139a012. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Bliven M. L., Holden H. E., Jr Effect of levamisole on the mitosis of murine thymocytes in culture. Immunopharmacology. 1979 Jun;1(3):245–254. doi: 10.1016/0162-3109(79)90041-9. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Chang Y. H. Comparative study of cyclophosphamide, 6-mercaptopurine, azathiopurine and methotrexate. Relative effects on the humoral and the cellular immune response in the mouse. Clin Exp Immunol. 1976 Nov;26(2):346–354. [PMC free article] [PubMed] [Google Scholar]
- Pirofsky B., Nolte M. T., Bardana E. J., Jr The effect of oxisuran on human immunological responsiveness. Transplantation. 1975 Nov;20(5):357–361. doi: 10.1097/00007890-197511000-00001. [DOI] [PubMed] [Google Scholar]
- Simonsen M. On the nature and measurement of antigenic strength. Transplant Rev. 1969;3:22–35. doi: 10.1111/j.1600-065x.1970.tb00253.x. [DOI] [PubMed] [Google Scholar]
- van Dijk H., Bakker I. A., Testerink J., Bloksma N., Willers J. M. Oxisuran and immune reactions: mediation of oxisuran action by the adrenal glands. J Immunol. 1975 Dec;115(6):1587–1591. [PubMed] [Google Scholar]


