Abstract
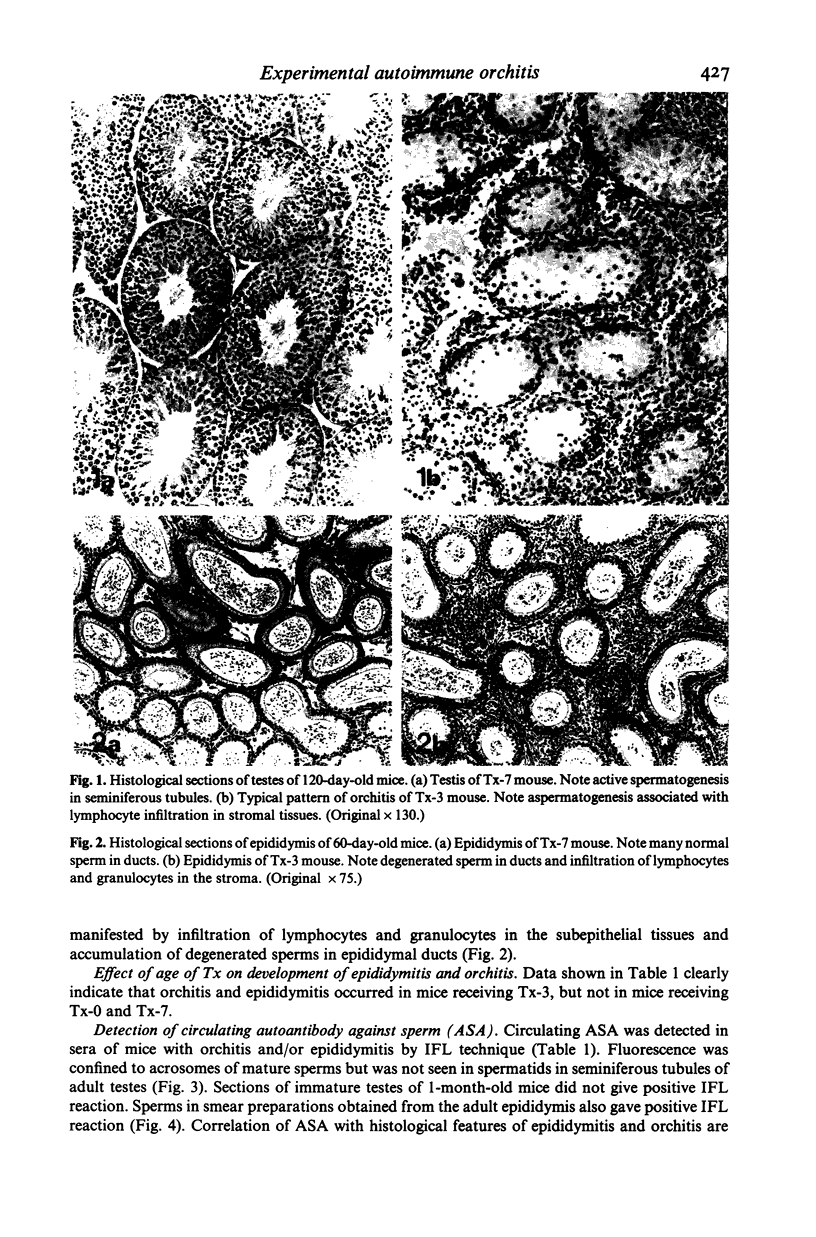
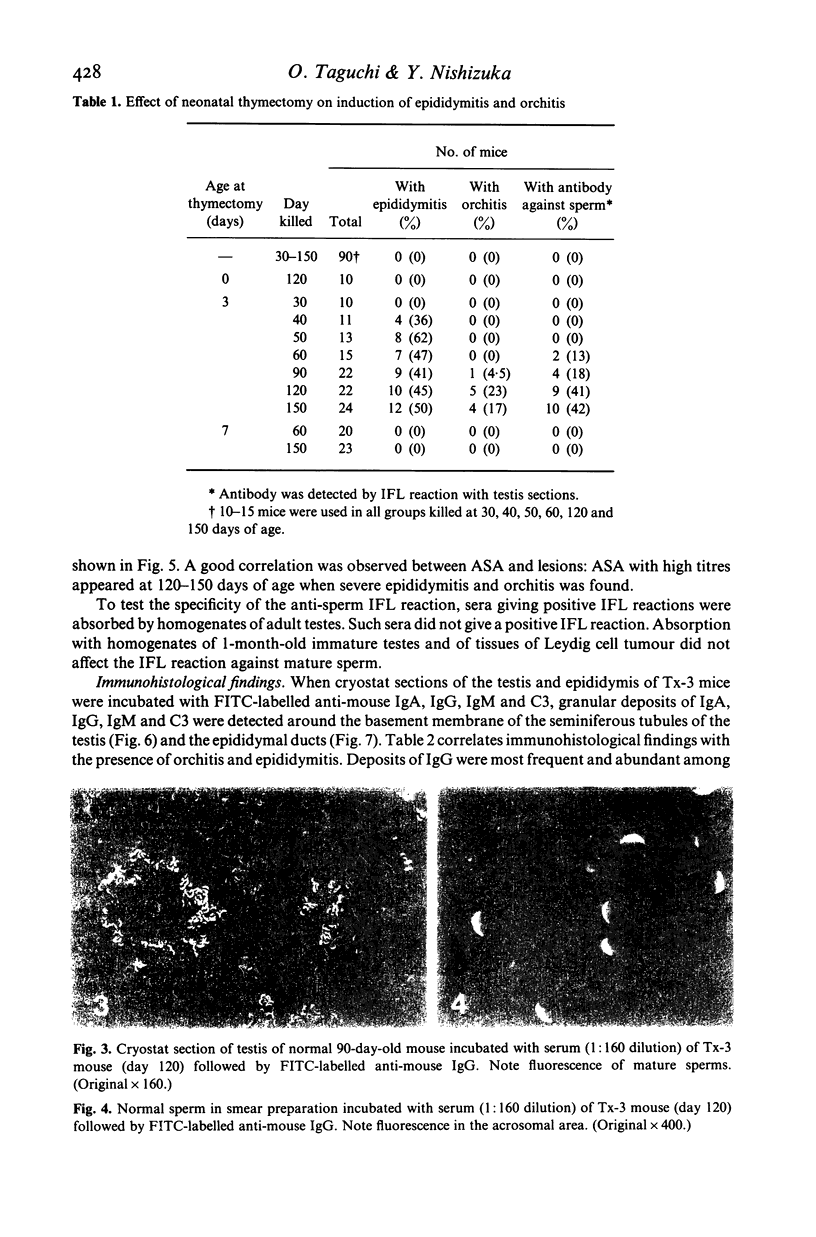
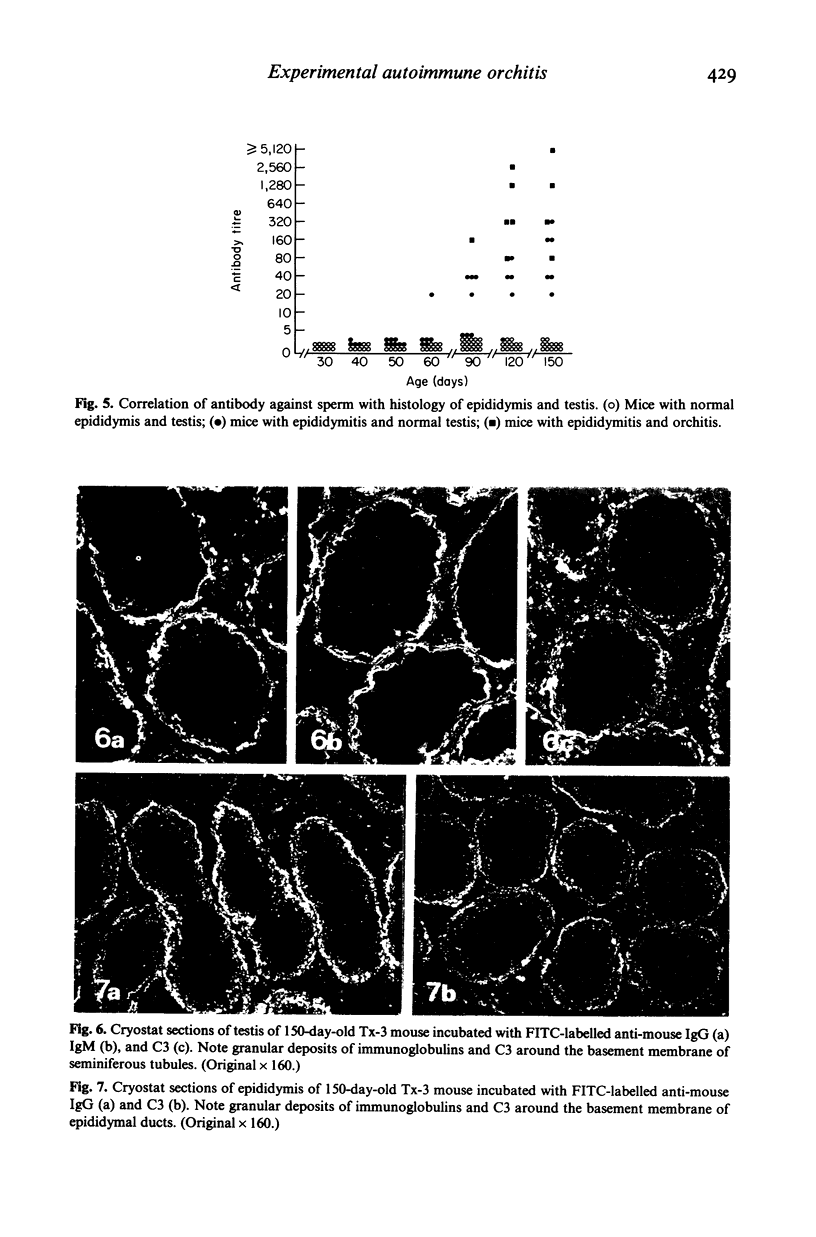
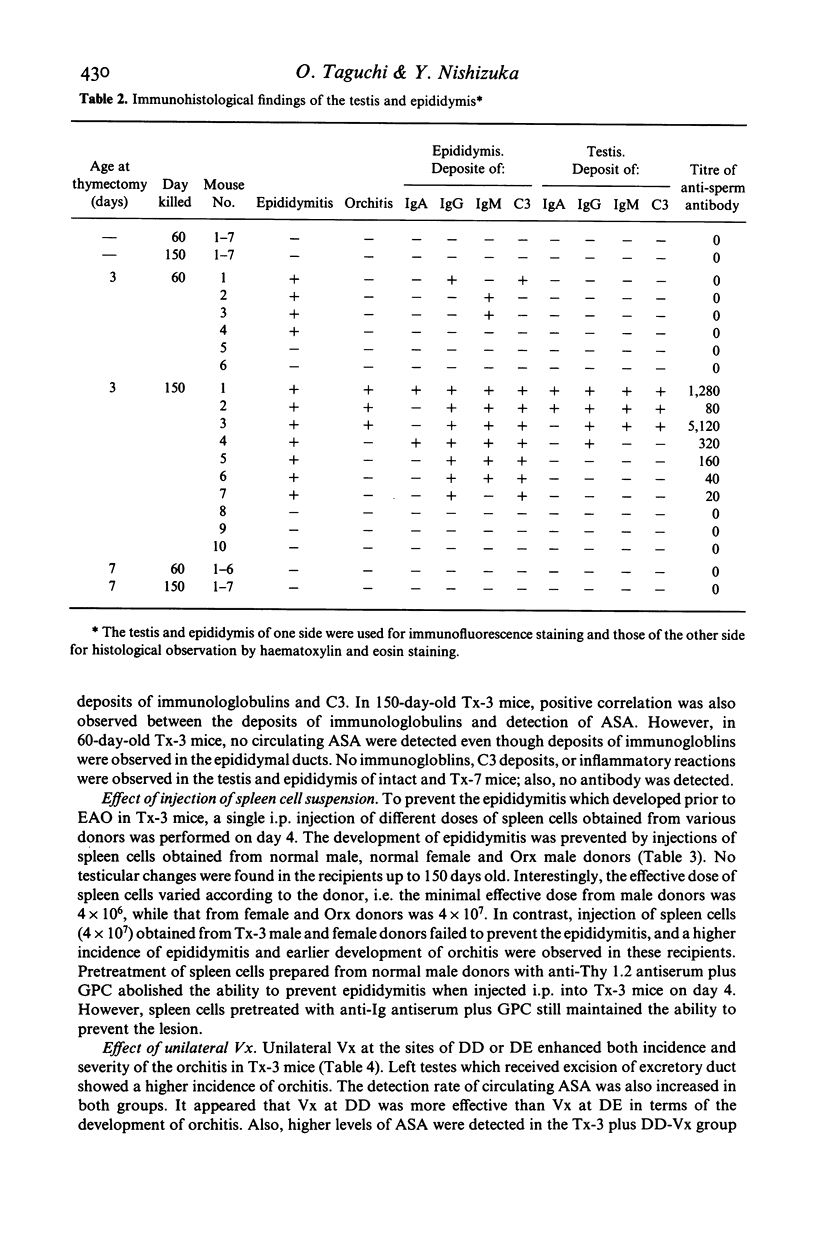
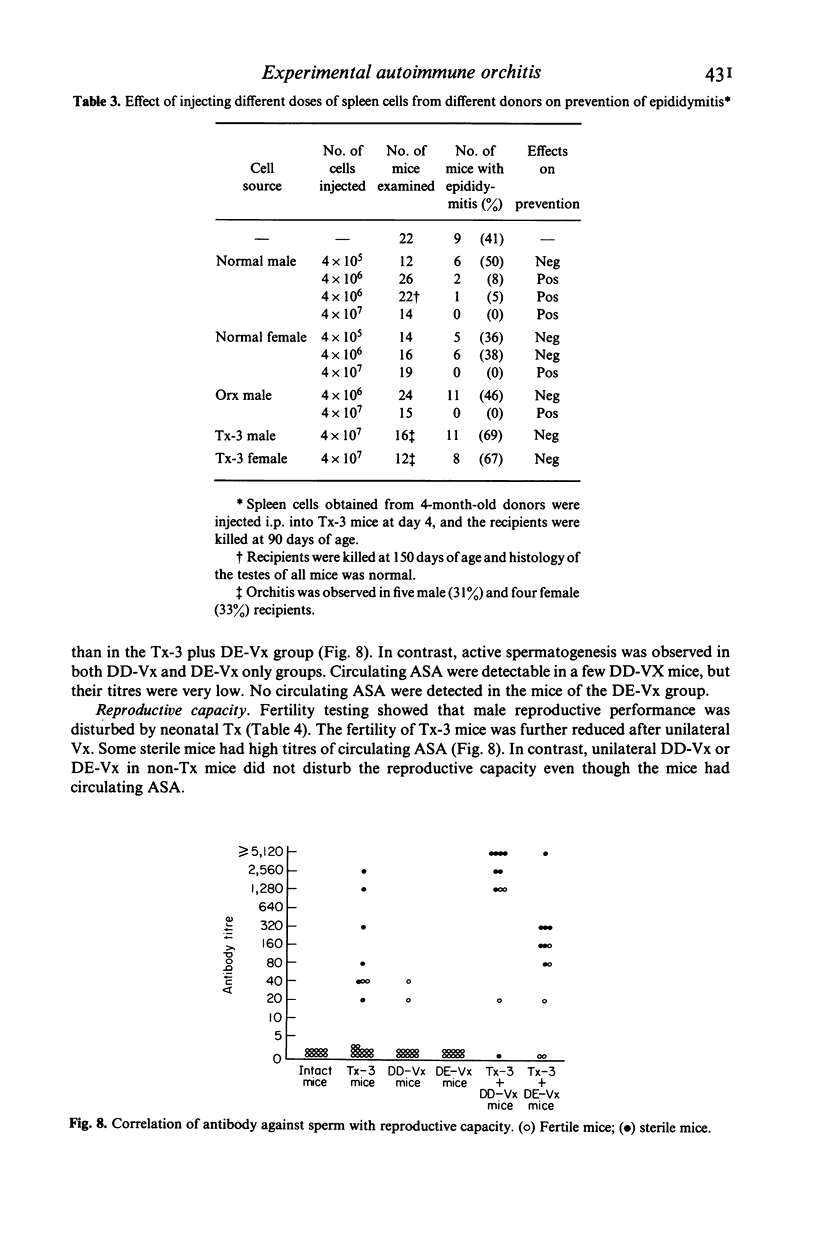
Experimental autoimmune orchitis (EAO) developed in (C57Bl/6Cr x A/JCr)F1 mice 3-4 months after neonatal thymectomy (Tx) without any sensitization. The age of Tx was critical for induction of EAO: Tx at day 3 (Tx-3) was effective, but Tx at day 0 or day 7 was not effective. This lesion resulted in atrophy of the testis and was characterized by disappearance of mature sperms, formation of multinuclear giant cells in seminiferous tubules and infiltration of lymphocytes in the stroma. Epididymitis was observed prior to the development of EAO. Presence of circulating autoantibody(s) against sperms (ASA) was demonstrated by indirect immunofluorescence. The acrosomal area of mature sperms, but not of immature spermatids, was strongly. The incidence of EAO and titre of ASA increased when Tx-3 mice were unilaterally vasectomized (Vx). The majority of mice with high titres of circulating ASA were sterile. Epididymitis and orchitis could be prevented in Tx-3 mice by injection of adult spleen cells on day 4. The most effective source was normal male. Spleen cells from normal female donors and day-0 orchidectomized (Orx) donors were less effective, while those of Tx-3 male and female donors failed to prevent epididymis and orchitis. The cell population in normal male spleen effective in preventing epididymitis was shown to be a T cell population (Thy 1+, Ig-) by experiments with respective antisera treatment. These results showed that sensitization with sperm autoantigen occurred in the epididymis after Tx-3, more efficiently after Tx-3 plus unilateral Vx, and that this autosensitization was prevented by a specific suppressor T cell population, which was present in normal males but absent in Tx-3 mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. J., Tung K. S. Immunological and morphological effects of vasectomy in the rabbit. Anat Rec. 1977 Jul;188(3):339–350. doi: 10.1002/ar.1091880307. [DOI] [PubMed] [Google Scholar]
- Ansbacher R. Sperm-agglutinating and sperm-immobilizing antibodies in vasectomized men. Fertil Steril. 1971 Oct;22(10):629–632. [PubMed] [Google Scholar]
- Bigazzi P. E., Kosuda L. L., Hsu K. C., Andres G. A. Immune complex orchitis in vasectomized rabbits. J Exp Med. 1976 Feb 1;143(2):382–404. doi: 10.1084/jem.143.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian A., Jackson J. J., Carlo D. J., Limjuco G. A., Eylar E. H. Experimental allergic aspermatogenic orchitis. III. Isolation of spermatozoal glycoproteins and their role in allergic aspermatogenic orchitis. J Immunol. 1975 Dec;115(6):1731–1743. [PubMed] [Google Scholar]
- Johnson M. H., Setchell B. P. Protein and immunoglobulin content of rete testis fluid of rams. J Reprod Fertil. 1968 Nov;17(2):403–406. doi: 10.1530/jrf.0.0170403. [DOI] [PubMed] [Google Scholar]
- Kojima A., Taguchi O., Nishizuka Y. Experimental production of possible autoimmune castritis followed by macrocytic anemia in athymic nude mice. Lab Invest. 1980 Apr;42(4):387–395. [PubMed] [Google Scholar]
- Kojima A., Tanaka-Kojima Y., Sakakura T., Nishizuka Y. Prevention of postthymectomy autoimmune thyroiditis in mice. Lab Invest. 1976 Jun;34(6):601–605. [PubMed] [Google Scholar]
- Kojima A., Tanaka-Kojima Y., Sakakura T., Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976 Jun;34(6):550–557. [PubMed] [Google Scholar]
- Nishizuka Y., Sakakura T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology. 1971 Sep;89(3):886–893. doi: 10.1210/endo-89-3-886. [DOI] [PubMed] [Google Scholar]
- Rümke P., Titus M. Spermagglutinin formation in male rats by subcutaneously injected syngeneic epididymal spermatozoa and by vasoligation or vasectomy. J Reprod Fertil. 1970 Feb;21(1):69–79. doi: 10.1530/jrf.0.0210069. [DOI] [PubMed] [Google Scholar]
- Sakakura T., Nishizuka Y. Thymic control mechanism in ovarian development: reconstitution of ovarian dysgenesis in thymectomized mice by replacement with thymic and other lymphoid tissues. Endocrinology. 1972 Feb;90(2):431–437. doi: 10.1210/endo-90-2-431. [DOI] [PubMed] [Google Scholar]
- Samuel T., Kolk A. H., Rümke P., Van Lis J. M. Autoimmunity to sperm antigens in vasectomized men. Clin Exp Immunol. 1975 Jul;21(1):65–74. [PMC free article] [PubMed] [Google Scholar]
- Shulman S. Antigenicity and autoimmunity in sexual reproduction: a review. Clin Exp Immunol. 1971 Sep;9(3):267–288. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y. Autoimmune oophoritis in thymectomized mice: T cell requirement in adoptive cell transfer. Clin Exp Immunol. 1980 Nov;42(2):324–331. [PMC free article] [PubMed] [Google Scholar]
- Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980 Jun;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- Toullet F., Voisin G. A., Nemirovsky M. Histoimmunochemical localization of three guinea-pig spermatozoal autoantigens. Immunology. 1973 Apr;24(4):635–653. [PMC free article] [PubMed] [Google Scholar]
- Voisin G. A., Toullet F. Etude sur l'orchite aspermatogénétique autoimmune et les autoantigènes de spermatozoides chez le cobaye. Ann Inst Pasteur (Paris) 1968 Jun;114(6):727–755. [PubMed] [Google Scholar]




