Abstract
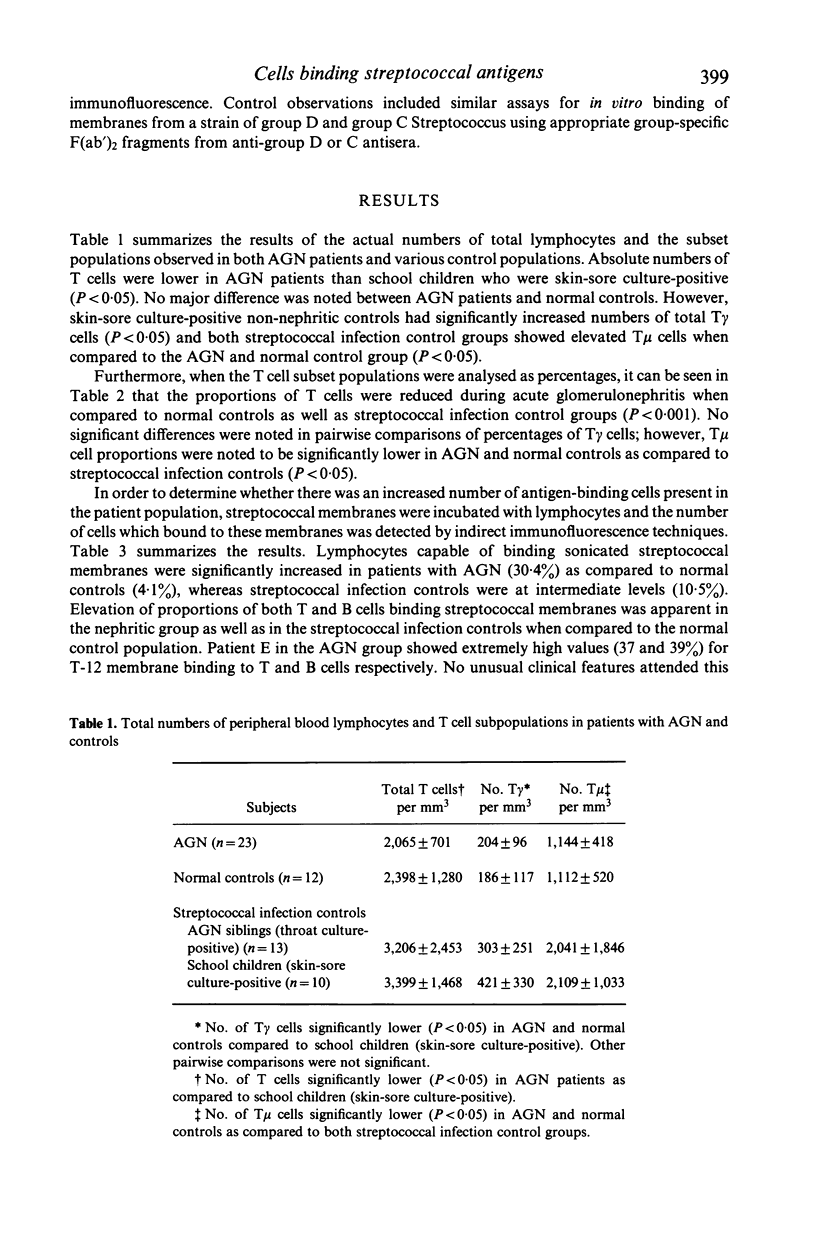
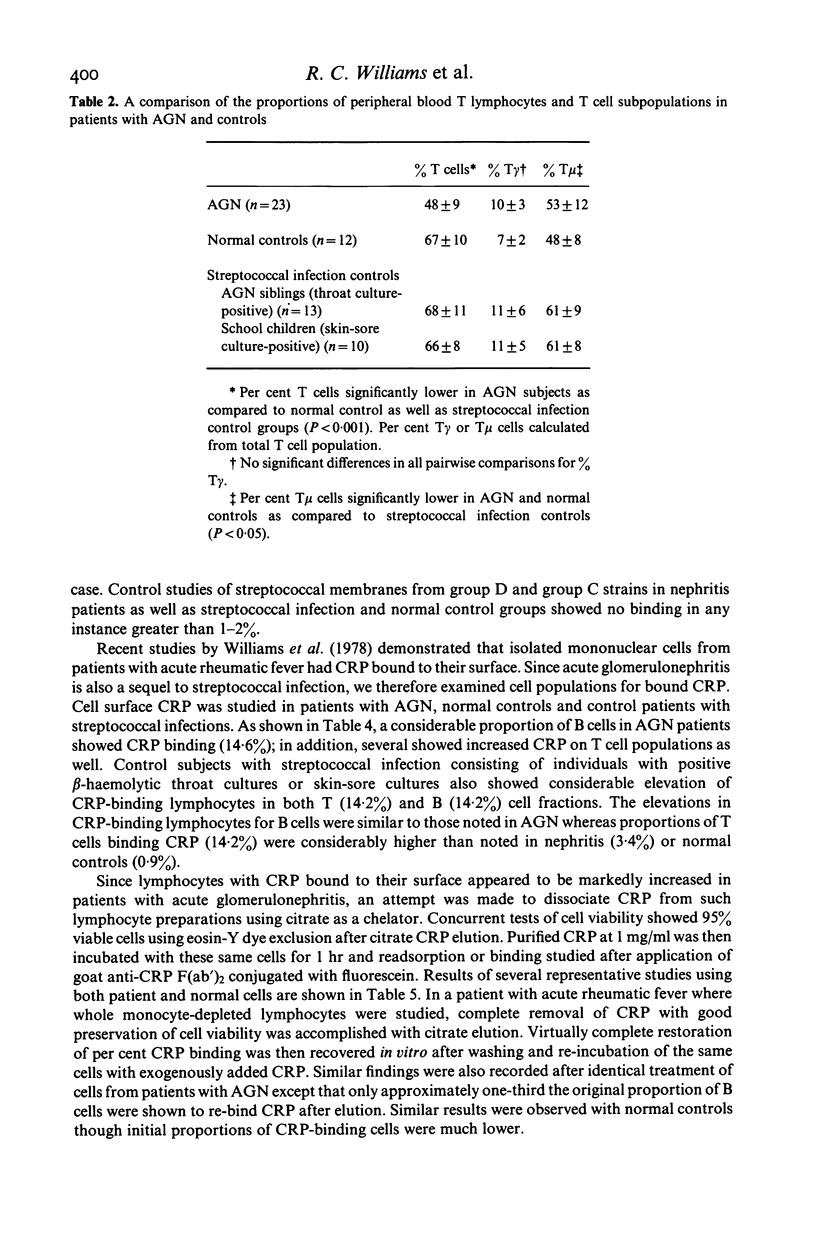
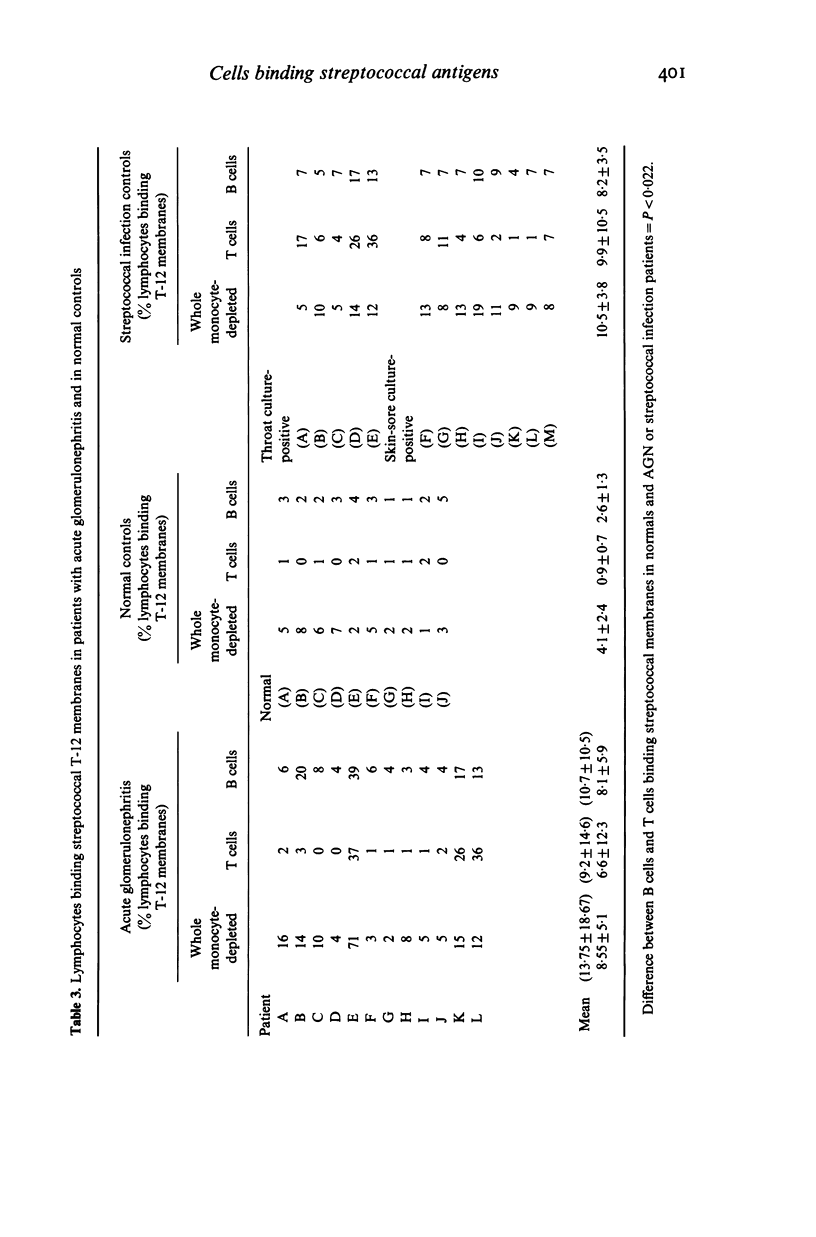
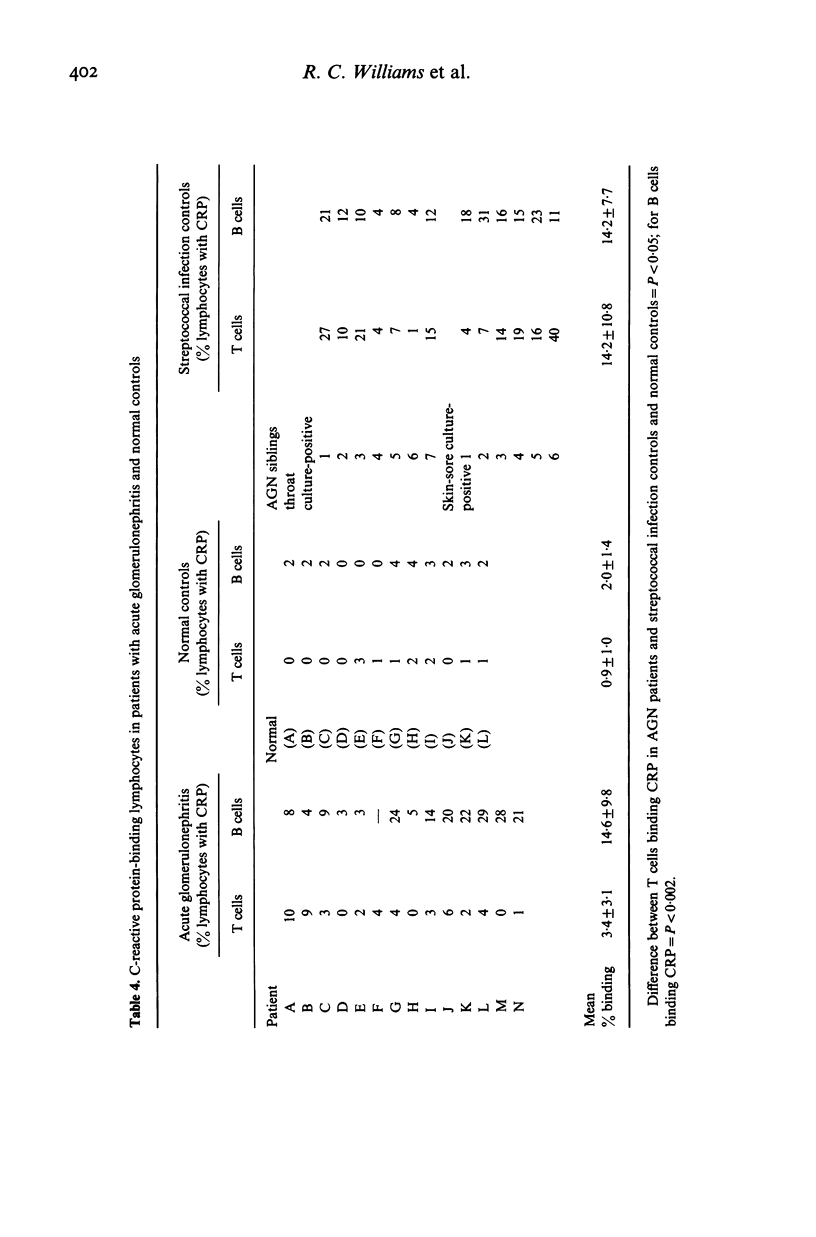
T lymphocyte surface markers were examined in 23 patients with acute post-streptococcal glomerulonephritis (AGN) in parallel with normal controls and individuals without nephritis who showed evidence of pharyngeal or skin-sore beta-haemolytic streptococcal infection. Numbers of T gamma cells were similar in AGN and normal controls but were significantly lower (P less than 0.05) than those in skin-sore culture-positive streptococcal infection controls. Numbers of T mu cells were similar in AGN and normal controls but were lower (P less than 0.05) than those observed in streptococcal controls. Percentages of T mu cells were similar in AGN and normal controls but were lower (P less than 0.05) than those recorded in streptococcal infection control groups. Proportions of T cells were reduced during AGN (P less than 0.05). Lymphocytes capable of binding type 12 group A streptococcal membranes were increased (30.4%) in patients with AGN as compared to normal controls (4.1%). Subjects with streptococcal infection alone showed elevated but intermediate relative numbers (10.5%) of lymphocytes binding group A membranes. Increased relative numbers of both B and T lymphocytes binding group A streptococcal membranes were present in both AGN and non-nephritogenic streptococcal infection controls.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat J. G., Gombos E. A., Baldwin D. S. Depressed cellular immune response to streptococcal antigens in poststreptococcal glomerulonephritis. Clin Immunol Immunopathol. 1977 Mar;7(2):230–239. doi: 10.1016/0090-1229(77)90050-2. [DOI] [PubMed] [Google Scholar]
- Bhat J. G., Gombos E. A., Baldwin D. S. Depressed leukocyte migration inhibition in response to streptococcal antigens in poststreptococcal glomerulonephritis. Clin Immunol Immunopathol. 1980 May;16(1):48–56. doi: 10.1016/0090-1229(80)90165-8. [DOI] [PubMed] [Google Scholar]
- Christensen P., Burova L. A., Grubb A., Grubb R., Samuelsson G., Schalén C., Svensson M. L. Interaction of the Fc part of IgG with Lancefield extracts of hemolytic streptococci. Strain specificity and activity. Acta Pathol Microbiol Scand C. 1979 Feb;87C(1):73–77. [PubMed] [Google Scholar]
- Christensen P., Sjöholm A. G., Holm S., Hovelius B., Mårdh P. A. Binding of aggregated IgG in the presence of fresh serum by group A streptococci producing pharyngeal infection: possible connection with types frequently involved in acute nephritis. Acta Pathol Microbiol Scand B. 1978 Feb;86(1):29–33. doi: 10.1111/j.1699-0463.1978.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Cudkowicz G., Shearer G. M., Ito T. Cellular differentiation of the immune system of mice. VI. Strain differences in class differentiation and other properties of marrow cells. J Exp Med. 1970 Oct 1;132(4):623–635. doi: 10.1084/jem.132.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon H. C., Jr, Reeves M. S. Streptococcal immune responses in nephritis after skin infections. Am J Med. 1974 Mar;56(3):333–346. doi: 10.1016/0002-9343(74)90615-9. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Janossy G., Roberts M., Rapson N. T., Ellis R. B., Chessels J., Lister T. A., Catovsky D. Membrane phenotyping: diagnosis, monitoring and classification of acute 'lymphoid' leukaemias. Haematol Blood Transfus. 1977;20:61–75. doi: 10.1007/978-3-642-66639-1_7. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T., Bankhurst A. D., Williams R. C., Jr Studies of T- and B-cell interactions in adult patients with combined immunodeficiency. J Clin Invest. 1980 Feb;65(2):422–431. doi: 10.1172/JCI109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Holmberg O., Kronvall G. Immunoglobulin-binding structure on bovine group G streptococci different from type III Fc receptors on human group G streptococci. Infect Immun. 1979 Jan;23(1):1–7. doi: 10.1128/iai.23.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissenson A. R., Baraff L. J., Fine R. N., Knutson D. W. Poststreptococcal acute glomerulonephritis: fact and controversy. Ann Intern Med. 1979 Jul;91(1):76–86. doi: 10.7326/0003-4819-91-1-76. [DOI] [PubMed] [Google Scholar]
- Potter E. V., Siegel A. C., Simon N. M., McAninch J., Earle D. P., Poon-King T., Mohommed I., Abidh S. Streptococcal infections and epidemic acute glomerulonephritis in South Trinidad. J Pediatr. 1968 Jun;72(6):871–884. doi: 10.1016/s0022-3476(68)80445-7. [DOI] [PubMed] [Google Scholar]
- SEEGAL B. C., ANDRES G. A., HSU K. C., ZABRISKIE J. B. STUDIES ON THE PATHOGENESIS OF ACUTE AND PROGRESSIVE GLOMERULONEPHRITIS IN MAN BY IMMUNOFLUORESCEIN AND IMMUNOFERRITIN TECHNIQUES. Fed Proc. 1965 Jan-Feb;24:100–108. [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G., Connell M. S., Priore R. L. Cellular differentiation of the immune system of mice. I. Separate splenic antigen-sensitive units for different types of anti-sheep antibody-forming cells. J Exp Med. 1968 Sep 1;128(3):437–457. doi: 10.1084/jem.128.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Barratt T. M., Hayward A. R., Soothill J. F. The inhibition of complement-dependent lymphocyte rosette formation by the sera of children with steroid-sensitive hephrotic syndrome and other renal diseases. Clin Exp Immunol. 1975 Aug;21(2):236–243. [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Kilpatrick K. A., Kassaby M., Abdin Z. H. Lymphocytes binding C-reactive protein during acute rheumatic fever. J Clin Invest. 1978 May;61(5):1384–1393. doi: 10.1172/JCI109056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Zabriskie J. B., Mahros F., Hassaballa F., Abdin Z. H. Lymphocyte surface markers in acute rheumatic fever and post-streptococcal acute glomerulonephritis. Clin Exp Immunol. 1977 Jan;27(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, van de Rijn I., Mahros F., Abdin Z. H., Reid H., Poon-King T. Lymphocytes binding C-reactive protein and streptococcal membranes in acute rheumatic fever. J Lab Clin Med. 1980 Nov;96(5):803–814. [PubMed] [Google Scholar]
- van de Rijn I., Fillit H., Brandeis W. E., Reid H., Poon-King T., McCarthy M., Day N. K., Zabriskie J. B. Serial studies on circulating immune complexes in post-streptococcal sequelae. Clin Exp Immunol. 1978 Dec;34(3):318–325. [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Chemical analysis of changes in membrane composition during growth of Streptococcus pyogenes. Infect Immun. 1979 Dec;26(3):883–891. doi: 10.1128/iai.26.3.883-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]


