Abstract
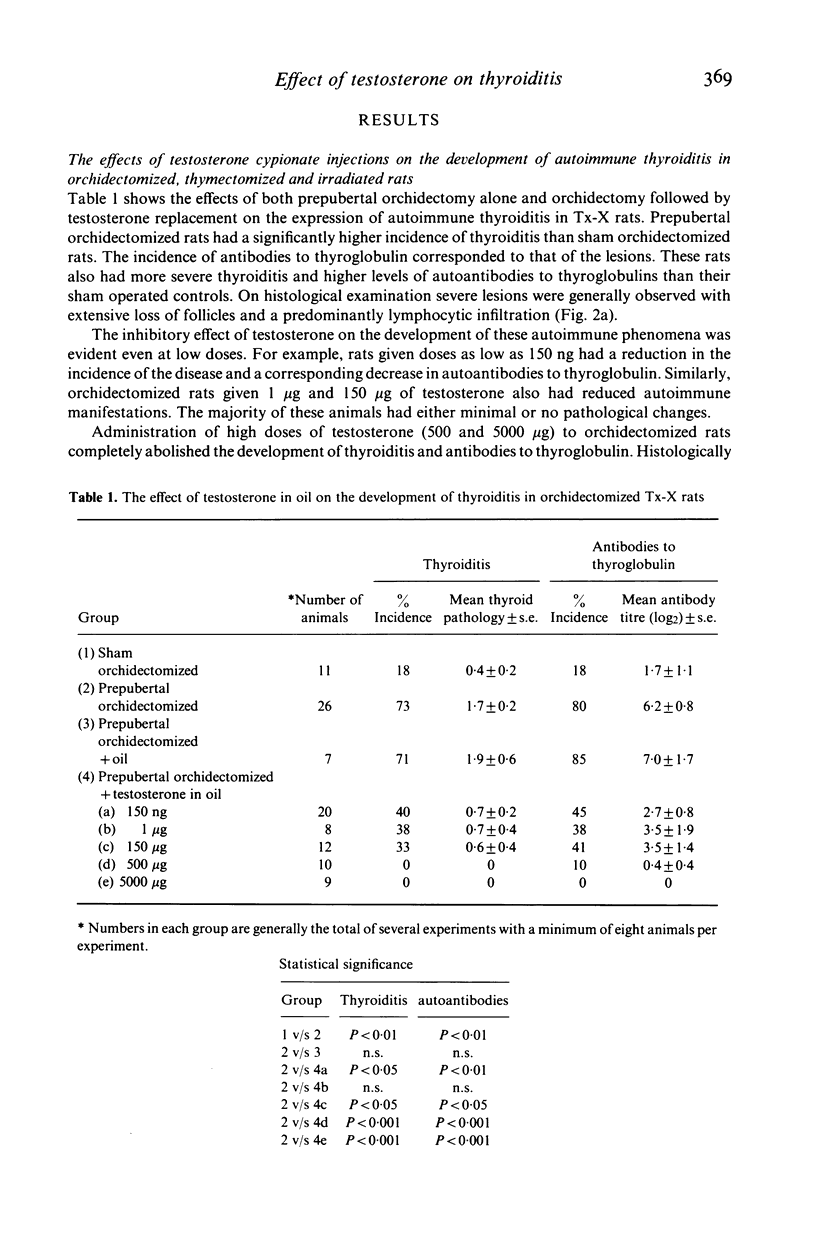
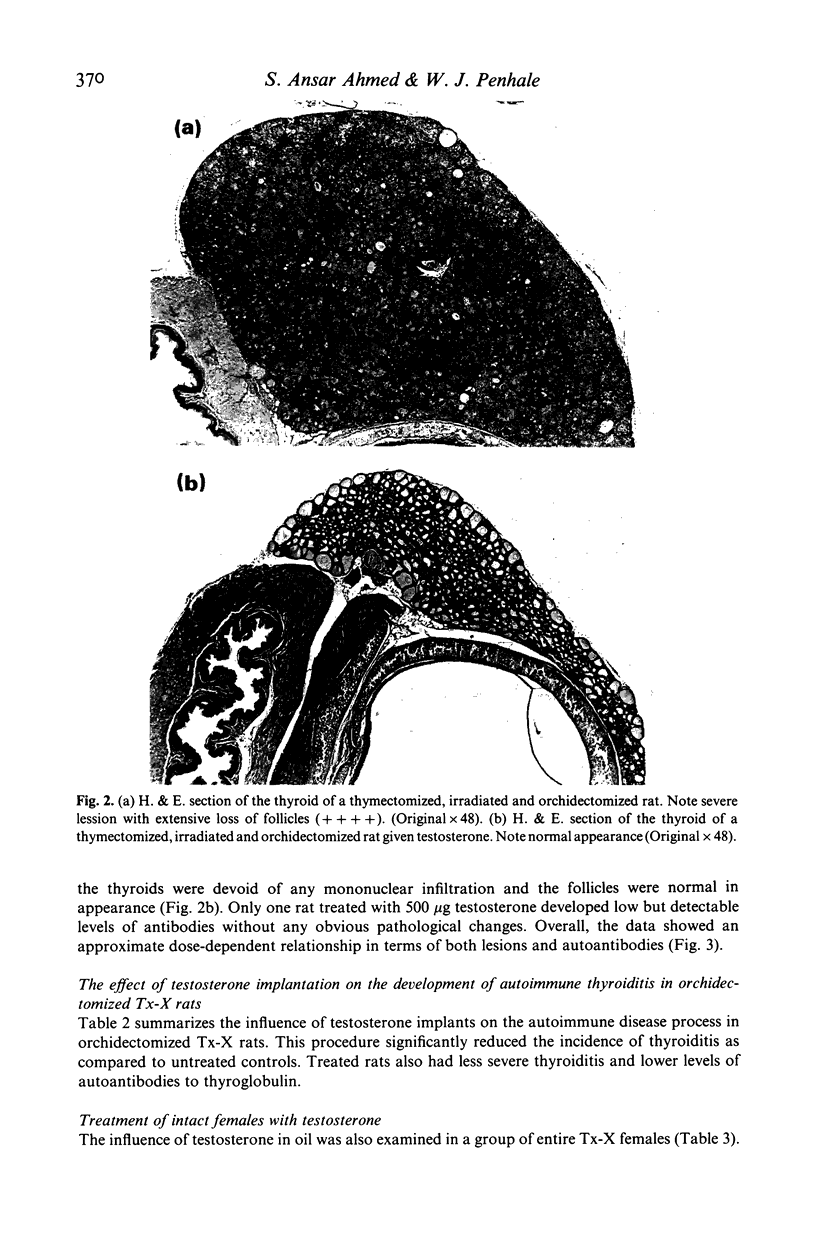
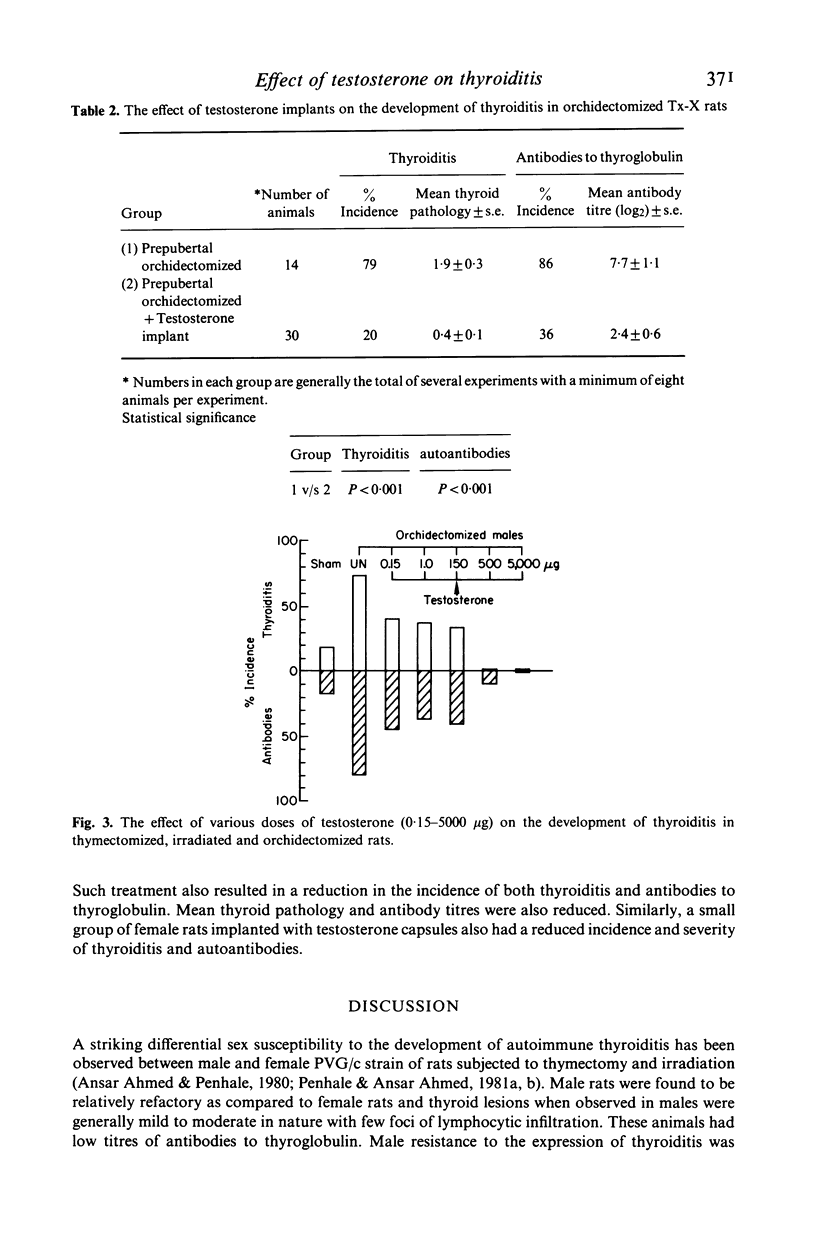
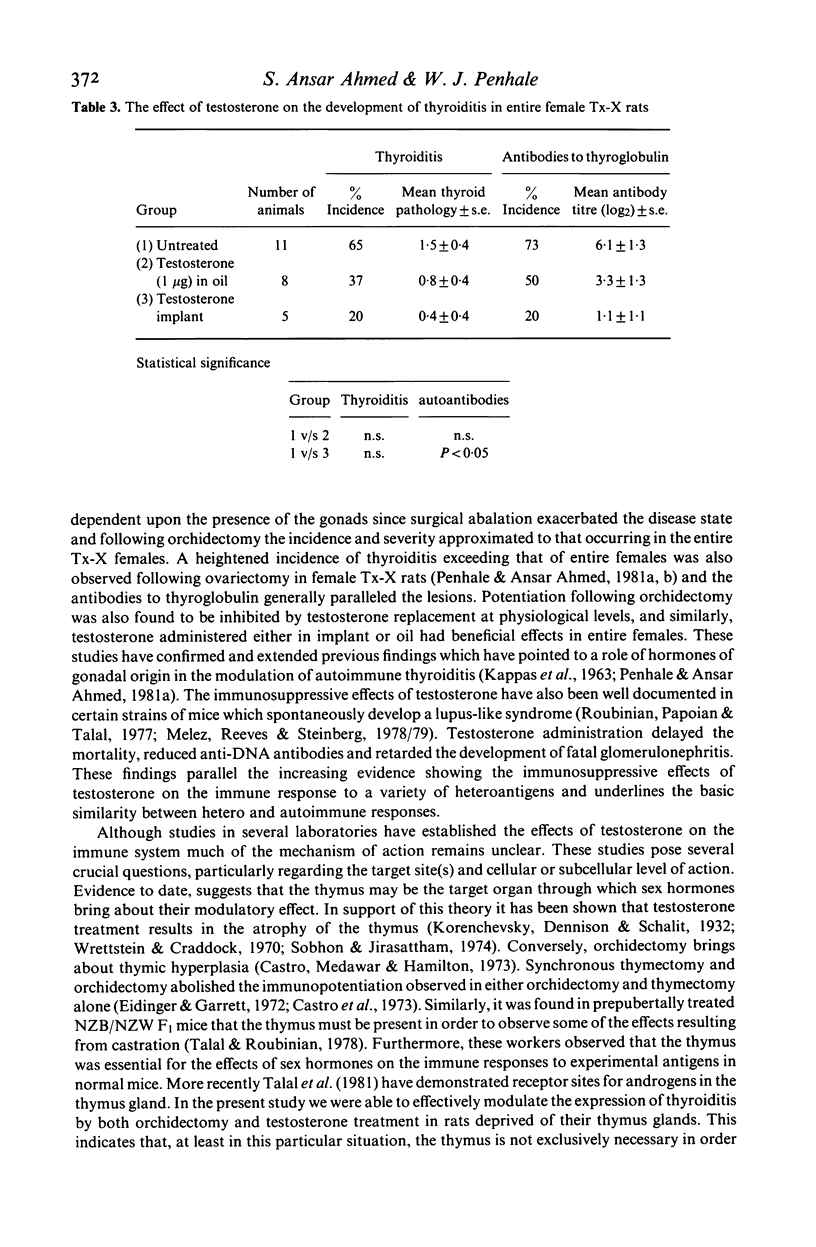
Orchidectomy was found to potentiate the development of autoimmune thyroiditis induced by thymectomy and irradiation (Tx-X) in male PVG/c strain rats. Conversely, testosterone administration to orchidectomized Tx-X rats markedly reduced or inhibited the development of this condition. When given in varying quantities by injection in oil over a period of 15 weeks the inhibitory effect on the development of both thyroiditis and thyroglobulin autoantibodies was found to be directly related to dose. Levels between 150 ng and 150 micrograms/100 gm body weight reduced the incidence and severity of the disease whilst levels of 500 micrograms and 5000 micrograms abrogated these autoimmune effects. Testosterone in implant form had a similar effect. Low doses of testosterone administered by either procedure were also found to be beneficial to entire female Tx-X rats. These results indicate that sex steroid hormones have an important modulatory influence on the genesis of autoimmune thyroiditis. Furthermore, it is also apparent in this particular model that this influence can be demonstrated in the absence of the thymus gland
Full text
PDF



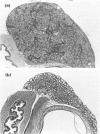
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. A., Penhale W. J. Thyroid transplantation developing autoimmune thyroiditis following thymectomy and irradiation. Clin Exp Immunol. 1981 Sep;45(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Berjivich S., Ressel M. Effect of sex on susceptibility of adult mice to coxsackie B1 virus infection. Arch Gesamte Virusforsch. 1967;22(1):246–251. doi: 10.1007/BF01240519. [DOI] [PubMed] [Google Scholar]
- Brent L., Medawar P. Quantitative studies on tissue transplantation immunity. 8. The effects of irradiation. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):413–423. doi: 10.1098/rspb.1966.0074. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigen G. A., Fraser R. C., Peterson N. S. Sex hormones and the immune response. II. Perturbation of antibody production by estradiol 17beta. Int Arch Allergy Appl Immunol. 1978;57(6):488–497. doi: 10.1159/000232143. [DOI] [PubMed] [Google Scholar]
- Frey-Wettstein M., Craddock C. G. Testosterone-induced depletion of thymus and marrow lymphocytes as related to lymphopoiesis and hematopoiesis. Blood. 1970 Mar;35(3):257–271. [PubMed] [Google Scholar]
- Friedman S. B., Grota L. J., Glasgow L. A. Differential susceptibility of male and female mice to encephalomyocarditis virus: effects of castration, adrenalectomy, and the administration of sex hormones. Infect Immun. 1972 May;5(5):637–644. doi: 10.1128/iai.5.5.637-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff R. J., Lappé M. A., Snell G. D. The influence of the gonads and adrenal glands on the immune response to skin grafts. Transplantation. 1969 Feb;7(2):105–111. doi: 10.1097/00007890-196902000-00003. [DOI] [PubMed] [Google Scholar]
- Healy J. W., Leeman S. Hemolysin response of rats treated neonatally with steroids. Endocrinology. 1967 Apr;80(4):762–764. doi: 10.1210/endo-80-4-762. [DOI] [PubMed] [Google Scholar]
- KALTER S. S., SMOLIN H. J., McELHANEY J. M., TEPPERMAN J. Endocrines and their relation to influenza virus infection. J Exp Med. 1951 Jun;93(6):529–538. doi: 10.1084/jem.93.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongshavn P. A., Bliss J. Q. Sex differences in survival of H-2 incompatible skin grafts in mice treated with antithymocyte serum. Nature. 1970 May 2;226(5244):451–451. doi: 10.1038/226451a0. [DOI] [PubMed] [Google Scholar]
- MADDOCK R. K., Jr INCIDENCE OF SYSTEMIC LUPUS ERYTHEMATOSUS BY AGE AND SEX. JAMA. 1965 Jan 11;191:137–138. doi: 10.1001/jama.1965.03080020065026. [DOI] [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., McKenna R. P., Irvine W. J. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin Exp Immunol. 1973 Oct;15(2):225–236. [PMC free article] [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., Urbaniak S. J., Irvine W. J. Susceptibility of inbred rat strains to experimental thyroiditis: quantitation of thyroglobulin-binding cells and assessment of T-cell function in susceptible and non-susceptible strains. Clin Exp Immunol. 1975 Jan;19(1):179–191. [PMC free article] [PubMed] [Google Scholar]
- Reilly R. W., Thompson J. S., Bielski R. K., Severson C. D. Estradiol-induced wasting syndrome in neonatal mice. J Immunol. 1967 Feb;98(2):321–330. [PubMed] [Google Scholar]
- Rifkind D., Frey J. A. Sex difference in antibody response of CFW mice to Candida albicans. Infect Immun. 1972 May;5(5):695–698. doi: 10.1128/iai.5.5.695-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinian J. R., Papoian R., Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977 Jun;59(6):1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhon P., Jirasattham C. Effect of sex hormones on the thymus and lymphoid tissue of ovariectomized rats. Acta Anat (Basel) 1974;89(2):211–225. doi: 10.1159/000144285. [DOI] [PubMed] [Google Scholar]
- Terres G., Morrison S. L., Habicht G. S. A quantitative difference in the immune response between male and female mice. Proc Soc Exp Biol Med. 1968 Mar;127(3):664–667. doi: 10.3181/00379727-127-32768. [DOI] [PubMed] [Google Scholar]
- WASHBURN T. C., MEDEARIS D. N., Jr, CHILDS B. SEX DIFFERENCES IN SUSCEPTIBILITY TO INFECTIONS. Pediatrics. 1965 Jan;35:57–64. [PubMed] [Google Scholar]
- WILLIAMS E. D., DONIACH I. The post-mortem incidence of focal thyroiditis. J Pathol Bacteriol. 1962 Jan;83:255–264. doi: 10.1002/path.1700830127. [DOI] [PubMed] [Google Scholar]