Abstract
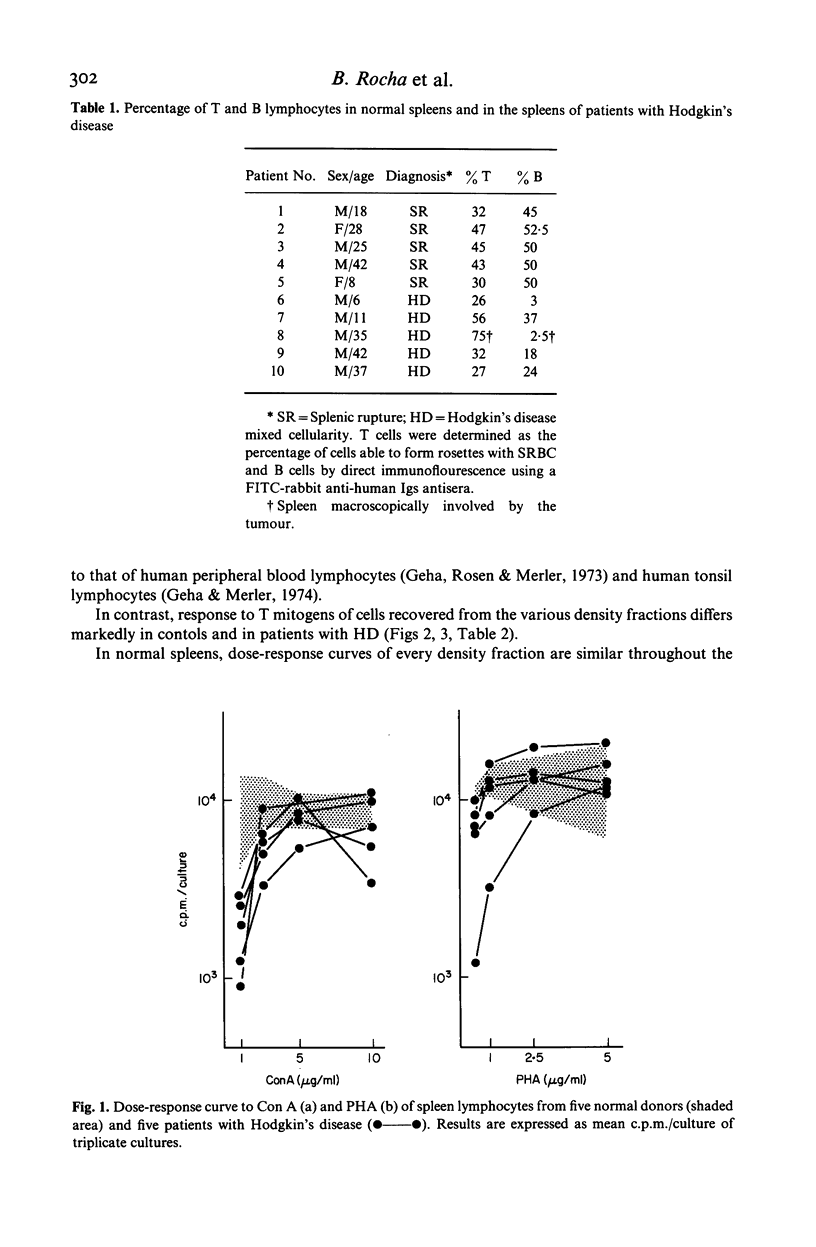
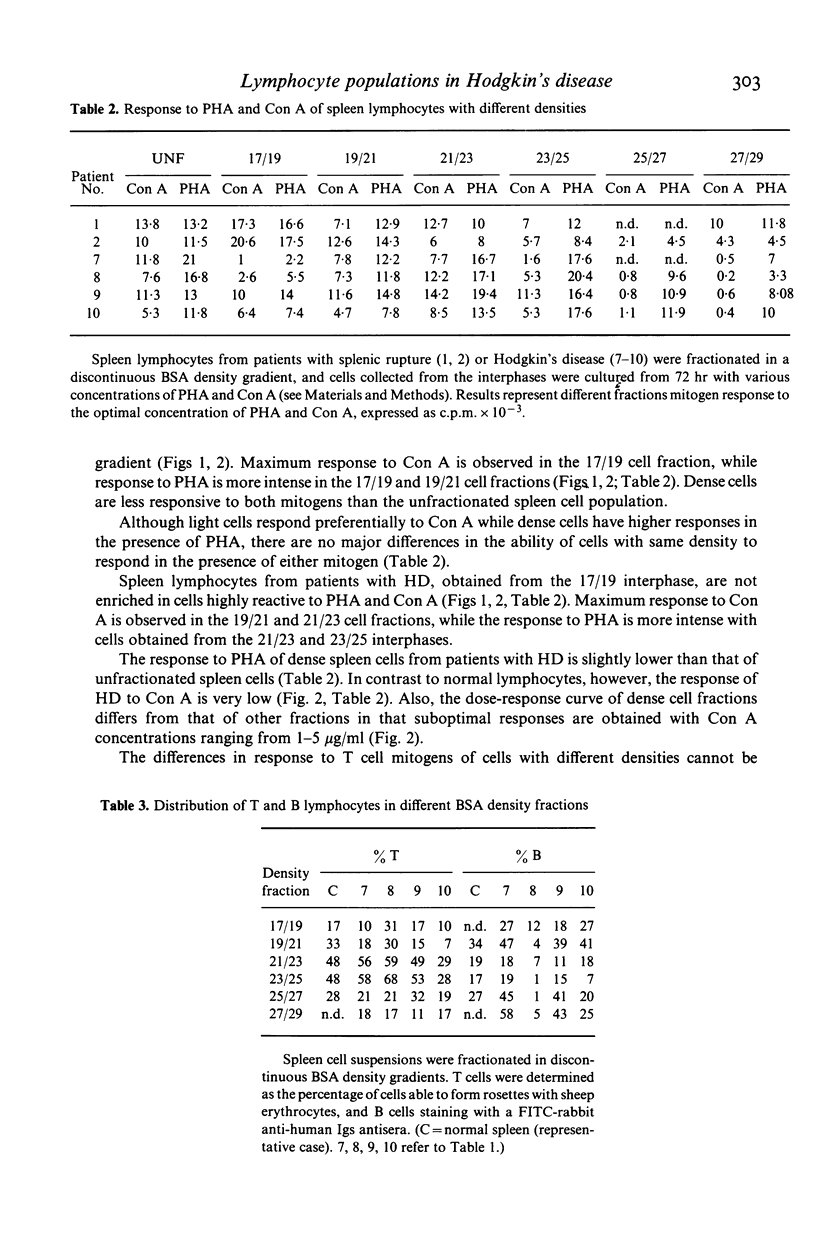
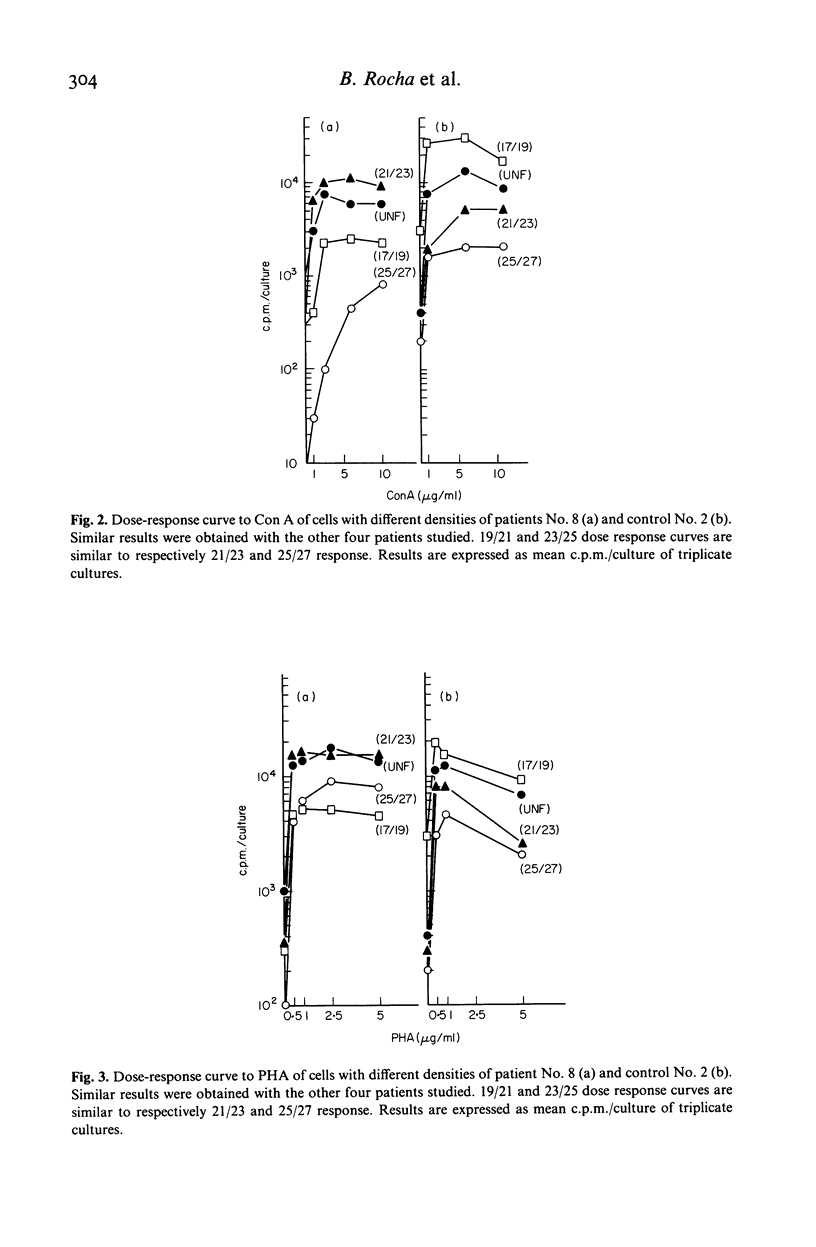
Spleen lymphocytes from five patients with Hodgkin's disease (HD) type mixed cellularity and five normal controls were studied. An increased percentage of 'null cells' was observed in three out of the five patients studied. The two other patients had increased percentage of T lymphocytes. Lymphocytes from spleen with HD had a decreased ability to respond to suboptimal concentrations of T cell mitogens. When these cells were fractionated in a discontinuous density gradient a subpopulation of cells, unable to respond to Con A, was recovered from the dense cell fractions. The implications of the present findings to the understanding of the physiopathological changes in HD are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AISENBERG A. C. Studies on delayed hypersensitivity in Hodgkin's disease. J Clin Invest. 1962 Nov;41:1964–1970. doi: 10.1172/JCI104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisenberg A. C., Long J. C. Lymphocyte surface characteristics in malignant lymphoma. Am J Med. 1975 Mar;58(3):300–306. doi: 10.1016/0002-9343(75)90595-1. [DOI] [PubMed] [Google Scholar]
- Christian C. L. Editorial: Systemic lupus erythematosus and type C RNA viruses. N Engl J Med. 1976 Aug 26;295(9):501–502. doi: 10.1056/NEJM197608262950911. [DOI] [PubMed] [Google Scholar]
- Dicke K. A., Tridente G., van Bekkum D. W. The selective elimination of immunologically competent cells from bone marrow and lymphocyte cell mixtures. 3. In vitro test for detection of immunocompetent cells in fractionated mouse spleen cell suspensions and primate bone marrow suspensions. Transplantation. 1969 Oct;8(4):422–434. doi: 10.1097/00007890-196910000-00014. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Response of human thymus-derived (T) and non-thymus-derived (B) lymphocytes to mitogenic stimulation in vitro. Eur J Immunol. 1974 Mar;4(3):193–199. doi: 10.1002/eji.1830040308. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Rosen F. S., Merler E. Identification and characterization of subpopulations of lymphocytes in human peripheral blood after fractionation on discontinuous gradients of albumin. The cellular defect in X-linked agammaglobulinemia. J Clin Invest. 1973 Jul;52(7):1726–1734. doi: 10.1172/JCI107354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni V., Del Giacco G. S., Manconi P. E., Tognella S., Mantovani G. Letter: Lymphocytes in spleen in Hodgkin's disease. Lancet. 1975 Feb 8;1(7902):332–333. doi: 10.1016/s0140-6736(75)91238-6. [DOI] [PubMed] [Google Scholar]
- Hunter C. P., Pinkus G., Woodward L., Moloney W. C., Churchill W. H. Increased T lymphocytes and IgMEA-receptor lymphocytes in Hodgkin's disease spleens. Cell Immunol. 1977 Jun 15;31(2):193–198. doi: 10.1016/0008-8749(77)90020-x. [DOI] [PubMed] [Google Scholar]
- Häyry P., Roberts P. J., Ranki A., Weber T. Selective responses of mouse T lymphocytes to different T mitogens and in mixed lymphocyte culture induced cytolysis reaction. Cell Immunol. 1976 Jul;25(1):121–128. doi: 10.1016/0008-8749(76)90102-7. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. R., Belpomme D. Letter: T and B lymphocytes in spleen in Hodgkin's disease. Lancet. 1975 Mar 29;1(7909):747–748. doi: 10.1016/s0140-6736(75)91660-8. [DOI] [PubMed] [Google Scholar]
- KELLY W. D., LAMB D. L., VARCO R. L., GOOD R. A. An investigation of Hodgkin's disease with respect to the problem of homotransplantation. Ann N Y Acad Sci. 1960 May 31;87:187–202. doi: 10.1111/j.1749-6632.1960.tb23192.x. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. Mechanism of T cell activation. I. A screening of "step one" ligands. Eur J Immunol. 1980 Feb;10(2):93–99. doi: 10.1002/eji.1830100205. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Iscove N. N., Coutinho A. Two distinct factors are required for induction of T-cell growth. Nature. 1980 Feb 14;283(5748):664–666. doi: 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- Levy R., Kaplan H. S. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. doi: 10.1056/NEJM197401242900402. [DOI] [PubMed] [Google Scholar]
- Resnick G. D., Nachman R. L. Reed-Sternberg cells in Hodgkin's disease contain fibronectin. Blood. 1981 Feb;57(2):339–342. [PubMed] [Google Scholar]
- Sousa M. D., Yang M., Lopes-Corrales E., Tan C., Hansen J. A., Dupont B., Good R. A. Ecotaxis: the principle and its application to the study of Hodgkin's disease. Clin Exp Immunol. 1977 Jan;27(1):143–151. [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E. Functional heterogeneity of murine lymphoid cells. 3. Differential responsiveness of T cells to phytohemagglutinin and concanavalin A as a probe for T cell subsets. J Immunol. 1973 Feb;110(2):362–375. [PubMed] [Google Scholar]
- Wagstaff J., Gibson C., Thatcher N., Ford W. L., Sharma H., Crowther D. Human lymphocyte traffic assessed by indium-111 oxine labelling: clinical observations. Clin Exp Immunol. 1981 Mar;43(3):443–449. [PMC free article] [PubMed] [Google Scholar]
- Whisler R. L., Stobo J. D. Heterogeneity of murine regulatory T cells. I. Subpopulations of amplifier and suppressor T cells. J Exp Med. 1976 Aug 1;144(2):398–413. doi: 10.1084/jem.144.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]


