Abstract
Sex specificity of neural mechanisms modulating nociceptive information has been demonstrated in rodents, and these qualitative sex differences appear to be relevant to analgesia from κ-opioid receptor agonists, a drug class reported to be clinically effective only in women. Via quantitative trait locus mapping followed by a candidate gene strategy using both mutant mice and pharmacological tools, we now demonstrate that the melanocortin-1 receptor (Mc1r) gene mediates κ-opioid analgesia in female mice only. This finding suggested that individuals with variants of the human MC1R gene, associated in our species with red hair and fair skin, might also display altered κ-opioid analgesia. We found that women with two variant MC1R alleles displayed significantly greater analgesia from the κ-opioid, pentazocine, than all other groups. This study demonstrates an unexpected role for the MC1R gene, verifies that pain modulation in the two sexes involves neurochemically distinct substrates, and represents an example of a direct translation of a pharmacogenetic finding from mouse to human.
The documentation of sex differences in pain and analgesic sensitivity has been an active endeavor in recent years (1). Increasing evidence suggests that such sex differences may reflect the activation of sex-specific neural mechanisms (2–4). For example, we reported a sexual dimorphism in the ability of the noncompetitive N-methyl-d-aspartate (NMDA) antagonist, MK-801 (dizocilpine), to reverse stress-induced analgesia (SIA) produced by forced cold-water swims in mice (4). Although both sexes displayed equipotent analgesia, only in males was this analgesia blocked by MK-801, thus implying that females possess a distinct, non-NMDAergic system. Additional research by ourselves and others revealed that the female-specific system is enabled by circulating estrogen (4, 5). However, estrogen appears to be permissive rather than modulating, because females are MK-801 insensitive at all stages of the estrous cycle (6). To shed light on the genetic and neurochemical basis of sex-specific analgesia, we used quantitative trait locus (QTL) mapping to investigate swim SIA in both sexes of a (C57BL/6J × DBA/2J)F2 intercross (B6D2F2) (7). QTL mapping is a technique in which the coinheritance of continuous traits and polymorphic DNA markers (e.g., microsatellites or single nucleotide polymorphisms) can be used to broadly identify the chromosomal location of genes responsible for trait variability (8). Distal mouse chromosome 8 [>52 centimorgans (cM)] was found to be linked to (i.e., coinherited with) SIA in female mice with a logarithm of odds (LOD) score >6.0, but was unlinked in male mice. Named Siafq1, the QTL accounted for 17–26% of the overall variance in the SIA of females.
This same sexual dimorphism (i.e., NMDA mediation in males but not females) was also found to characterize κ-opioid analgesia (9), implying that the two phenomena have a common substrate. Thus, in the present study, we investigated the possible sex specificity of the genetic mediation of κ-opioid analgesia in mice. Three convergent lines of evidence point to the mouse melanocortin-1 receptor gene (Mc1r) as the female-specific QTL. Given that clinically used analgesics with activity at the κ-opioid receptor have been reported to be more effective in women than men (10–13), we then investigated the functional effects of variants at the human analogue of this gene on one such drug, pentazocine.
Materials and Methods
Mouse Nociceptive Testing.
Nociceptive sensitivity was measured with the 49°C hot-water tail-withdrawal assay. Mice were lightly restrained in a cloth/cardboard holder, and the distal half of their tails was immersed in water thermostatically controlled at 49 ± 0.2°C. The latency to vigorous removal of the tail from the water was measured to the nearest 0.1 s with a stopwatch. At each time point, two latency determinations (separated by 10 s) were made and averaged. A cut-off latency of 15 s was used to prevent the possibility of tissue damage. Procedures were approved by local animal care and use committees.
U50,488 Analgesia.
Naïve DBA/2J (D2), C57BL/6J (B6), or C57BL/6J-Mc1re (e/e) mice (6–8 weeks of age) of both sexes were tested for baseline sensitivity as described above, injected with U50,488 (10–70 mg/kg, i.p.; in 10 ml/kg saline), and retested at 15, 30, and 60 min postinjection. For each mouse, analgesia was expressed as percent of total possible analgesia, by comparing the area under the time × latency curve (using the trapezoidal rule) to that of a hypothetical subject displaying cut-off latencies at all postinjection time points. This conservative method corrects for variability in individual baseline latencies. Expressing analgesia with respect to peak postinjection latencies yielded virtually identical results (data not shown). Half-maximal antinociceptive doses (AD50s) were calculated from percent analgesia data by using the method of Tallarida and Murray (14).
In some experiments, mice were pretreated (immediately before U50,488 injections) with either MK-801 (0.075 mg/kg, s.c.) or saline (10 ml/kg). This dose has been shown to effectively antagonize SIA in males, without motoric side effects (4). We have been unable to find a nonlethal MK-801 dose that significantly and consistently blocks SIA or κ-opioid analgesia in females of these strains.
QTL Mapping.
Genomic DNA was isolated from postmortem B6D2F2 mouse spleen tissue by phenol/chloroform extraction. Samples from five mice were discarded because of poor DNA yield. PCR was performed in a volume of 25 μl by using a standard hot-start protocol. PCR products were resolved on 7% nondenaturing polyacrylamide gels and visualized by laser detection of ethidium bromide. B6D2F2 mice were scored, by reference to progenitor strain samples run concurrently, for their genotype (B6/B6 homozygote, B6/D2 heterozygote, or D2/D2 homozygote) at three distal chromosome 8 microsatellite markers: D8Mit11 (49 cM), D8Mit360 (59 cM), and D8Mit56 (73 cM). Both sexes displayed expected Mendelian ratios of genotype at each marker.
Data from female and male mice were analyzed separately by using interval mapping as implemented by mapmaker/qtl (15). This software program, using maximum-likelihood estimation, iteratively calculates the statistical probability for genetic linkage between the trait and chromosomal locations at and between the markers used. The existence, broad location, and effect size of QTLs are thus assessed.
MC1R Antagonist Synthesis and in Vitro Assays.
The peptide Ac-Nle-Asp-Trp-DPhe-Nle-Trp-Lys-NH2 (16) was synthesized manually by solid-phase methodology using standard fluorenylmethoxycarbonyl strategy (see Supporting Materials and Methods, which are published as supporting information on the PNAS web site, www.pnas.org).
The compound has high potency as an antagonist at the MC1R with a pA2 = 8.4 in the frog skin assay (16), but with agonist activities at the human MC3R, MC4R, and MC5R with IC50s of 260 ± 82 nM, 60 ± 20 nM, and 910 ± 140 nM, respectively (unpublished data).
MC1R Antagonist Study.
Naïve Crl:CD-1 (ICR, St. Constant, Canada) mice (6–8 weeks of age) of both sexes were used in this experiment. Mice were tested for baseline sensitivity as described above, then given an intracerebroventricular (2.5 μl per mouse) injection of Ac-Nle-Asp-Trp-DPhe-Nle-Trp-Lys-NH2 (20 μg per mouse) or vehicle (10% DMSO) by the method of Laursen and Belknap (17). Immediately thereafter, they were injected with either MK-801 (0.075 mg/kg, s.c.) or saline (10 ml/kg). Fifteen minutes later all mice were tested for nociceptive sensitivity again, and then given 70 mg/kg (i.p.) U50,488. Mice were tested at 15, 30, and 60 min post-U50,488. Analgesia was quantified as described above, with reference to the second baseline. Data were analyzed by three-way ANOVA, followed by t tests where appropriate. Full dose–response curves could not be compiled because of limited peptide availability.
Human Study.
Forty-two healthy, non-Hispanic white volunteers, ages 18–41 (mean 25.4 years), were studied (12 male and nine female nonredheads, 12 male and nine female redheads) in two experimental sessions, which were conducted 3–5 days apart. In each session, thermal and ischemic pain testing was conducted before and 15 min after i.v. bolus administration of either pentazocine (0.5 mg/kg) or saline, in double-blind, randomized fashion. All women were studied in their follicular phase (4–10 days postmenses), and equal proportions in each of the two hair color groups were using oral contraceptives. Data were analyzed by two-way ANOVA. All procedures were approved by the University of Florida's Institutional Review Board.
Thermal testing involved administration of brief, repetitive, suprathreshold thermal stimuli to assess temporal summation of thermal pain (18). Ten heat pulses at 52°C were applied to the right volar forearm, with target temperatures delivered for a duration of <1 s, with a 2.5-s interpulse interval during which the temperature of the contactor returned to a baseline of 40°C. Subjects rated the intensity of each thermal pulse on a numerical scale of 0–100. Because 28% of subjects terminated the procedure before completion of 10 trials, data from the first five trials were included in the analysis.
Ischemic arm pain was induced via the submaximal effort tourniquet procedure (19). The left arm was exsanguinated by elevating it above heart level for 30 s, then occluded with a standard blood pressure cuff inflated to 240 mm Hg. Subjects performed 20 handgrip exercises at 50% of their maximum grip strength. The time of first reported pain (pain threshold) and the time at which subjects requested to stop (pain tolerance) were recorded, and there was an uninformed time limit of 15 min. Every 60 s subjects rated the intensity and unpleasantness of their arm pain by using combined numerical-verbal descriptor scales.¶¶ Seven of 42 (16.7%) subjects reached the time limit before drug administration. To replace missing values created when subjects discontinued before the time limit, the last value was carried forward.
Human Genotyping.
The MC1R polymorphism detection strategy involved sequencing the 5′ two-thirds of the ORF, where all of the known variants occur except for D294H, which was specifically screened for (see Supporting Materials and Methods).
Results
Sex-Specific Linkage of Distal Chromosome 8 to κ-Opioid Analgesia.
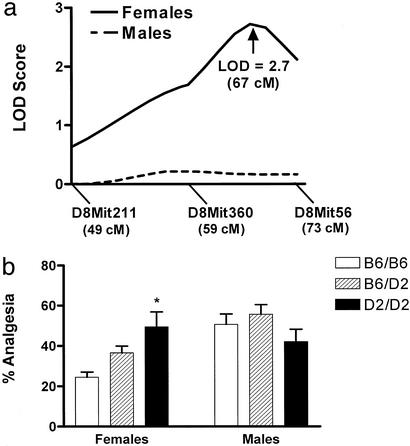
A total of 156 B6D2F2 mice (81 females; 75 males) were bred in our vivarium and tested for their antinociceptive sensitivity to systemic U50,488 (50 mg/kg, i.p.), a widely used κ-selective agonist. These same mice were later genotyped at three microsatellite markers spanning a 24-cM region on distal chromosome 8. As shown in Fig. 1a, significant linkage was observed in females (LOD = 2.7, P = 0.0019, 2 df), peaking at 67 cM (1 LOD drop-off confidence interval: 51 cM to end), with the QTL accounting for 25% of the overall trait variance. This LOD score exceeds the replication significance threshold suggested by Lander and Kruglyak (20) for a small genomic interval in which a QTL has previously been mapped. No evidence for linkage was obtained in males (LOD = 0.5, P = 0.32). Whereas female B6D2F2 mice inheriting two copies of the D2 allele at D8Mit56 (73 cM) displayed twice as much U50,488 analgesia as those inheriting two copies of the B6 allele, the allelic status of male mice at this marker was without consequence (Fig. 1b).
Figure 1.
Linkage of distal mouse chromosome 8 to U50,488 (50 mg/kg, i.p.) analgesia in female but not male B6D2F2 mice. (a) Male and female LOD score curves, showing the logarithm of the likelihood for a presence of a QTL at that location. A free genetic model yielded a peak LOD score of 2.7 in females; additive and recessive models also yielded significant LOD scores in this sex (2.3 and 2.4, respectively). The approximate locations of microsatellite markers used are shown below the ordinate. (b) Impact on U50,488 analgesia of inheritance of the three possible genotypes (B6/B6, homozygous for B6 allele; B6/D2, heterozygous; D2/D2, homozygous for D2 allele) at D8Mit56. The time course of U50,488 analgesia was identical in all groups (data not shown), peaking at 15 min postinjection. Bars represent mean ± SEM percent total analgesia (% analgesia) over the 60-min testing period. *, Significantly different from B6/B6 group, P < 0.05.
Neurochemical Mediation of κ-Opioid Analgesia in MC1R Mutant Mice.
Of the genes already mapped to the distal portion of mouse chromosome 8, one in particular emerged as a candidate gene for this QTL: Mc1r. The consensus location of Mc1r is 68 cM, only 1 cM away from the peak of linkage (syntenic human genome location: 16q24.3). Direct tests of Mc1r as a candidate gene for female-specific analgesia would involve blockade of MC1R function by genetic or pharmacological means.
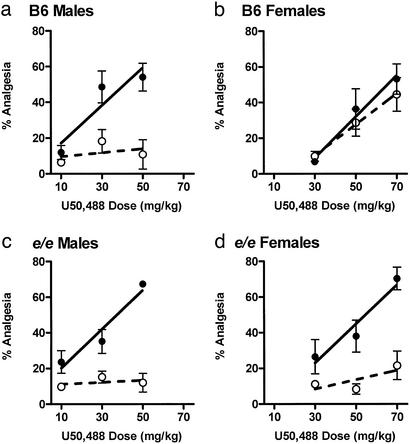
Known allelic variants of the Mc1r (formerly, extension) gene in mice include the tobacco (Mc1rE-tob), sombre (Mc1rE-so), and nonextension (Mc1re) alleles (21, 22). The latter variant is associated with a frameshift mutation in the second extracellular loop, such that the e/e (“recessive yellow”) mouse is a null mutant, having a completely nonfunctional Mc1r gene. Thus, the sex-specific involvement of MC1Rs in U50,488 analgesia could be evaluated by comparing e/e mice to the otherwise isogenic strain on which this mutation arose, B6. We therefore tested e/e, B6, and D2 mice (coprogenitors of the QTL mapping population) of both sexes for U50,488 analgesia (10–70 mg/kg, i.p.) in the presence and absence of MK-801. The observed analgesia was mediated by κ-opioid receptors in all populations, being completely blocked by the κ-selective antagonist nor-binaltorphimine (5 mg/kg, s.c.; administered 48 h before U50,488) in every case (data not shown). Although U50,488 can produce analgesia via actions in the periphery (23), the neural circuitry underlying the sex difference is almost certainly central, because in male mice MK-801 can block analgesia from U50,488 administered directly into the lateral ventricles (data not shown). In all three genotypes, saline-treated male mice were found to be modestly but significantly more sensitive to U50,488 analgesia than female mice (see Table 1 and Fig. 2). This quantitative sex difference is apparently related to circulating estrogen, because in B6 and e/e mice it was reversed by adult ovariectomy and reinstated by subsequent estrogen replacement therapy (see Fig. 5, which is published as supporting information on the PNAS web site). More importantly, the qualitative sex difference observed in D2 and B6 mice 1/m complete blockade of U50,488 analgesia by MK-801 in males, no effect in females 1/m was not seen in e/e mice. In this mutant strain, MK-801 was equally able to block U50,488 analgesia in both sexes (Fig. 2). That is, mutant e/e mice displayed male-like, NMDA-mediated U50,488 analgesia regardless of sex (Fig. 2) or hormonal status (see Fig. 5). Because the only known genetic difference between B6 females and e/e females is the absence of functional MC1Rs in the latter, we suggest that B6 females must be using MC1Rs to mediate U50,488 analgesia. The concurrent demonstration of genetic linkage of the trait to the genomic region containing Mc1r renders it highly unlikely that functional compensation by another protein is responsible for these findings.
Table 1.
Sensitivity of U50,488 analgesia in D2, B6, and e/e mice of both sexes to blockade by MK-801
| Genotype | Sex | Treatment | AD50,* mg/kg | Potency ratio,† sex | MK-801 block? |
|---|---|---|---|---|---|
| D2 | Male | Saline | 20.8 (15.3–28.4) | 1.7 (1.1–2.6)‡ | Yes |
| MK-801 | n.c. | ||||
| Female | Saline | 35.7 (28.6–44.6)§ | No | ||
| MK-801 | 46.8 (37.2–58.7) | ||||
| B6 | Male | Saline | 39.0 (27.3–55.6) | 1.7 (1.1–2.5)‡ | Yes |
| MK-801 | n.c. | ||||
| Female | Saline | 65.2 (53.5–79.6) | No | ||
| MK-801 | 82.4 (65.7–103.3) | ||||
| e/e | Male | Saline | 34.6 (26.4–45.5) | 1.6 (1.1–2.3)‡ | Yes |
| MK-801 | n.c. | ||||
| Female | Saline | 54.7 (43.4–69.0) | Yes | ||
| MK-801 | n.c. |
n.c., Not calculable; half-maximal analgesia was not obtained at any dose (see Fig. 2).
Half-maximal analgesic dose (AD50), calculated by the method of Tallarida and Murray (14). Confidence intervals of 95% are in parentheses.
Calculated as female AD50/male AD50 (saline groups only). Confidence intervals of 95% are in parentheses.
Males significantly more sensitive than corresponding female saline group, P < 0.05, based on potency ratio comparison with 1.0.
Significantly more sensitive than corresponding saline group in other genotypes, P < 0.05.
Figure 2.
Sex and genotype specificity of U50,488 analgesia dose–response relationships (10–70 mg/kg, i.p.), and the antagonistic effect of the NMDA antagonist, MK-801 (0.075 mg/kg, s.c.), in male and female mice with functional (B6, a and b) or nonfunctional (e/e, c and d) MC1Rs. Symbols (●, saline-treated; ○, MK-801-treated) represent mean ± SEM percent total analgesia (% analgesia) over the 60-min testing period. No differences were observed in baseline nociceptive sensitivity among genotypes (P = 0.12); males of both genotypes had modestly but significantly increased latencies relative to females (P < 0.005). n = 4–11 mice per genotype per sex per dose; these data have been replicated with equivalent sample sizes (not shown). See Table 1 for AD50 estimates calculated from these data.
Sex-Specific Effects of Pharmacological MC1R Blockade on κ-Opioid Analgesia.
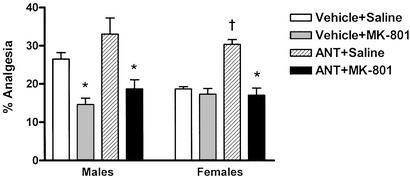
As a convergent line of evidence, we investigated the consequences of pharmacological MC1R blockade by using a potent and selective peptide antagonist, Ac-Nle-Asp-Trp-DPhe-Nle-Trp-Lys-NH2 (16). Based on the data obtained in e/e mutants, we hypothesized that antagonist treatment would also cause females to “switch” systems. As shown in Fig. 3, intracerebroventricular injection of the antagonist into outbred mice significantly potentiated U50,488 analgesia in females but not males. More importantly, the antagonist did indeed appear to cause a switch, reinstating the male-like MK-801 blockade in female mice. Similar results (data not shown) were obtained with another MC1R antagonist, c[Gly-Cpg-DNal(2′)-Arg-Trp-Glu-Val-Val-Gly-NH2] (cyclopentylglycine) (24), a compound with a Ki value at the human MC1R of 53 nM and 3.6-, 108- and 14.2-fold selectivity over human MC3Rs, MC4Rs, and MC5Rs, respectively.
Figure 3.
Effects of pharmacological blockade of MC1Rs by the peptide antagonist, Ac-Nle-Asp-Trp-DPhe-Nle-Trp-Lys-NH2 (ANT; 20 μg per mouse, i.c.v.), on the magnitude and MK-801 sensitivity of U50,488 analgesia in mice of both sexes. Bars represent mean ± SEM percent total analgesia (% analgesia) over the 60-min testing period. The outbred Crl:CD-1 mice used in this experiment were even less sensitive to U50,488 than C57BL/6 mice (see Fig. 2), but also displayed a quantitative sex difference in analgesic magnitude (P < 0.001). There was no evidence of analgesia from the ANT alone in either sex (data not shown). n = 4–6 mice per condition. These data have been replicated in a separate laboratory (not shown). *, Significantly lower than corresponding saline group, P < 0.001. †, Significantly higher than corresponding vehicle group, P < 0.001.
Sex-Specific Dependence of Pentazocine Analgesia on MC1R Status in Humans.
The best-known biological action of the MC1R is its role in the switching of melanin synthesis from the red/yellow phaeomelanin pathway to the black eumelanin pathway in both skin and hair (25). The majority of redheads are homozygotes or compound heterozygotes at three major MC1R variants (R151C, R160W, D294H) (26), and these variants produce loss of function (27, 28). Thus, human redheads are genetically analogous to e/e mice in this respect (but see ref. 29). Given that human sex differences in analgesic response to κ-opioid-preferring drugs have been reported (10–13), we tested men and women of different natural hair colors and skin types (see Table 2) for their sensitivity to one such drug, pentazocine, tested against experimental ischemic and thermal pain. Because the red hair/light skin phenotype can often occur independently of MC1R, we genotyped each subject as well.
Table 2.
Measures of pentazocine analgesia in humans by sex, hair, and skin phenotypes and MC1R genotype
| Sex | Hair color*
|
Skin type†
|
MC1R genotype‡
|
||||
|---|---|---|---|---|---|---|---|
| RH | NonRH | I & II | III & IV | Two variant alleles | 0/1 variant alleles | ||
| Number of subjects | Females | 9 | 9 | 11 | 7 | 5 | 13 |
| Males | 12 | 12 | 15 | 9 | 9 | 15 | |
| Δ Ischemic pain threshold§ | Females | 85.2 | 6.6 | 97.4 | −35.0 | 116.6 | 18.7 |
| (145.7) | (139.2) | (148.4) | (97.8) | (181.7) | (124.5) | ||
| Males | 17.4 | 98.3 | 20.2 | 120.6 | 8.8 | 87.3 | |
| (106.7) | (164.3) | (95.5) | (186.1) | (119.7) | (149.3) | ||
| Δ Ischemic pain tolerance¶ | Females | 237.0 | 62.0 | 233.3 | 24.8 | 408.0†† | 37.1 |
| (268.3) | (58.8) | (236.4) | (61.1) | (284.1) | (82.8) | ||
| Males | 62.3 | 84.6 | 84.6 | 59.6 | 46.4 | 84.1 | |
| (217.8) | (137.4) | (190.6) | (155.1) | (149.3) | (182.8) | ||
| Δ Sum ischemic pain intensity‖ | Females | 55.0 | 16.6 | 58.4†† | 0.3 | 84.8†† | 16.9 |
| (58.1) | (42.1) | (54.0) | (26.6) | (50.3) | (41.9) | ||
| Males | 20.9 | 40.3 | 25.4 | 39.3 | 18.7 | 37.8 | |
| (37.2) | (37.6) | (35.1) | (42.8) | (36.2) | (38.3) | ||
| Δ Sum ischemic pain unpleasantness** | Females | 46.1 | 11.1 | 54.4†† | −11.9 | 79.6†† | 9.0 |
| (60.0) | (51.8) | (55.6) | (31.9) | (45.7) | (49.6) | ||
| Males | 26.4 | 33.0 | 26.9 | 34.3 | 24.2 | 33.0 | |
| (48.1) | (44.4) | (43.3) | (51.1) | (41.0) | (49.0) | ||
| Δ Sum thermal pain intensity‡‡ | Females | −1.4 | −15.9 | 6.1 | −31.9 | 28.0†† | −22.8 |
| (56.1) | (38.9) | (46.9) | (41.1) | (48.9) | (40.1) | ||
| Males | 17.7 | 22.4 | 13.9 | 29.8 | 9.2 | 26.2 | |
| (48.8) | (61.9) | (46.4) | (66.6) | (51.0) | (56.5) | ||
Values presented are means (SD appears in parentheses). For all measures, greater values indicate more robust analgesia.
Redhead (RH) includes auburn (two) and strawberry (three) hair colors; NonRH includes blonde (five), brown (fourteen), and black (two). As seen by others previously (27), all subjects with two variant alleles were RH, but only 12 of 21 (57%) RH subjects had two variant alleles.
Based on Fitzpatrick skin type classifications: I: burn, never tan; II: burn, then tan; III: tan, sometimes burn; IV: tan, never burn. All subjects with two variant alleles had type I or type II skin.
Of the 14 subjects (29%) with two variant alleles, three were homozygous for R151C, one was homozygous for D294H, six were R151C/R160W compound heterozygotes, two were R151C/D294H compound heterozygotes, and one was a V92M/R160W compound heterozygote. Other than V92M, R151C, R160W, and D294H, the only nonconsensus allele observed was R163Q in one non-RH female. Observed allelic frequencies were very similar to published data (27).
Calculated as: (postdrug pain threshold − predrug pain threshold).
Calculated as: (postdrug pain tolerance − predrug pain tolerance).
Calculated as: (sum of all predrug ischemic pain intensity ratings − sum of all postdrug ischemic pain intensity ratings).
Calculated as: (sum of all predrug ischemic pain unpleasantness ratings − sum of all postdrug ischemic pain unpleasantness ratings).
Calculated as: (sum of predrug thermal pain intensity ratings trials 1–5 − sum of postdrug pain thermal intensity ratings trials 1–5).
Significantly higher than corresponding within-sex group, P < 0.05 (see text).
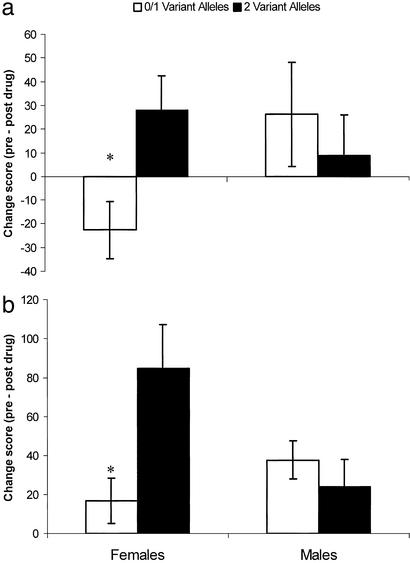
Pentazocine produced increases in ischemic pain thresholds and tolerance, decreases in ratings of ischemic pain intensity and unpleasantness, and decreases in thermal pain intensity ratings (all P < 0.05 relative to saline), indicating significant analgesia. No significant changes on any pain measure occurred after administration of saline, and there were no sex or group differences in the effects of saline on pain responses. For ischemic pain tolerance and ischemic pain ratings of intensity and unpleasantness, significant sex × genotype interactions were observed (P < 0.05); this interaction approached significance (P = 0.056) for thermal pain ratings. In all of these measures, significant effects of genotype emerged in women but not men. That is, our results (see Fig. 4) revealed a significant influence of MC1R genotype on analgesia in women only. Pentazocine at the dose used produced modest analgesia in all men. By contrast, “classic” light-skinned, redheaded women with two variant MC1R alleles displayed robust pentazocine analgesia against ischemic pain and were the only group to display convincing analgesia against thermal pain. Skin type appeared to be a better proxy for MC1R genotype than hair color, as these effects reached significance for ischemic pain when light- versus dark-skinned women were compared as described above, but did not quite do so for redheaded versus nonredheaded women (Table 2).
Figure 4.
Pentazocine analgesia by sex and MC1R genotype (two variant alleles vs. zero or one variant alleles) in humans. Bars represent mean (± SEM) difference scores for summed pain intensity ratings during each pain procedure (thermal pain, a; ischemic pain, b) were computed by subtracting postdrug from predrug ratings. No differences were observed in baseline pain responses or pain responses to saline administration across sex or genotype. *, Significantly different from other genotype within sex, P < 0.05.
Discussion
In the present study, QTL mapping of a biological trait was followed by functional studies testing a candidate gene hypothesis. The genetic linkage, mutant, and pharmacological results all were in support of a role for the mouse Mc1r gene, and in every case that role appeared to be specific to females. Formal proof that the Mc1r gene represents the female-specific QTL for U50,488 analgesia in the mouse would require positional cloning (i.e., the reduction of the confidence interval surrounding the QTL, eliminating all other genes on distal chromosome 8), identification of the relevant genetic variant, and perhaps a transgenic “rescue” experiment. Indeed, QTL mapping has been criticized based on the continuing difficulty of meeting this high burden of proof (30). QTL mapping has unique advantages over transgenic and mutagenesis approaches, however (31), and the value of functional studies in providing strong circumstantial evidence that particular genes represent QTLs is increasingly appreciated (32, 33). We would argue that because the ultimate goal of mouse genetics is the prediction of genetic effects in humans, much of this debate is beside the point in this case in light of our apparent success in translating this finding to our own species.
At present, a number of biological roles of MC1Rs are widely recognized: regulation of pigmentation and hair color in mammalian melanocytes; regulation of sun sensitivity, freckling, and skin cancer risk; and antiinflammatory effects via the attenuation of proinflammatory cytokine production (21, 22, 25, 26, 34). None of these functions appears to have any obvious relation to the inhibition of noninflammatory pain. However, in addition to their well-known localization in the periphery, MC1Rs are expressed in brain glial cells (see ref. 34) and neurons of the ventral periaqueductal gray (35), a brain area of critical relevance to the modulation of nociception (36). Thus, although the involvement of MC1Rs in analgesia is surprising, it is not inexplicable.
The identity of the relevant endogenous ligand of MC1R is as yet unclear. The most obvious candidate, of course, is the proopiomelanocortin gene product, α-melanocyte-stimulating hormone (α-MSH). α-MSH inhibition of thermal nociception has been demonstrated (37, 38), but other efforts have revealed an antiopioid role for α-MSH (e.g., ref. 39). The regulation of α-MSH release by κ-opioid receptors appears to be sexually dimorphic (40). We have, however, been unable to produce reliable effects on nociceptive sensitivity in either sex by using either α-MSH itself or a stable analog injected intracerebroventricularly in mice. Of great interest is the possibility that the endogenous κ-opioid receptor ligand, dynorphin, may be relevant, as dynorphin peptides (including des-Tyr fragments) can bind to melanocortin receptors, including MC1R, with nanomolar affinity (41). The direction of effect in the antagonist and human experiments (and a similar trend in e/e mice) suggests that MC1R activation by endogenous neuromodulators would exert antiopioid actions in females.
Also unresolved is the directionality of sex differences in κ-opioid analgesic magnitude. In laboratory rodents and even primates, males generally display greater κ-opioid analgesia, although this varies with genetic background (see ref. 42). Gear and colleagues (10–13), in contrast, have reported that women are more sensitive to the inhibition of molar extraction pain by a number of nonselective κ-opioid analgesics including pentazocine. We observed a trend in the opposite direction presently: toward greater analgesia in nonredheaded men than women. The discrepancy may reflect their use of an inflammatory pain model, the concurrent use of benzodiazepine sedatives, or the inclusion of a number of redheaded, fair-skinned women in their samples. It should also be noted that our mouse and human studies were performed exclusively in healthy, young adult subjects. Finally, we note that pentazocine, and other clinical analgesics with activity at κ-opioid receptors, do not bind selectively to this site, having affinity for μ-opioid receptor as well. Preliminary data (not shown) suggest that e/e mutants may have sex-specific changes in morphine analgesic magnitude; analogous human data would be of great interest. Regardless, the present findings suggest that sex differences in human pain inhibition may be caused by qualitatively different processing in men and women. The conservation of this genotypic effect from rodent to human is a promising indicator of the potential for mouse genetics to illuminate the basis of individual differences in humans and identify novel targets for analgesic development.
Supplementary Material
Acknowledgments
We thank Ian Robberechts, Claudia Campbell, Marisa Scott, and Beth Fisher for assistance with data collection, Dr. Wendy Sternberg for sharing some unpublished data, and Dr. Roger Cone for his generous gift of e/e mutant breeders. This work was supported with resources and the use of facilities at the Malcom Randall Veterans Affairs Medical Center (Gainesville, FL), by National Institutes of Health Grants DE12735 and DA15191 (to J.S.M.), DK17240 (to V.J.H.), and NS41670 (to R.B.F.), and by the Canada Research Chairs program and the Canada Foundation for Innovation (to J.S.M.).
Abbreviations
- α-MSH
α-melanocyte-stimulating hormone
- MC1R
melanocortin-1 receptor
- QTL
quantitative trait locus
- SIA
stress-induced analgesia
- LOD
logarithm of odds
- cM
centimorgan
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Coghill, R. C. & Gracely, R. H. (1996) Proc. Am. Pain Soc. 15, 86A.
References
- 1.Berkley K J. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 2.Liu N-J, Gintzler A R. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 3.Tershner S A, Mitchell J M, Fields H L. Pain. 2000;85:153–159. doi: 10.1016/s0304-3959(99)00257-2. [DOI] [PubMed] [Google Scholar]
- 4.Mogil J S, Sternberg W F, Kest B, Marek P, Liebeskind J C. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 5.Kavaliers M, Galea L A M. Pain. 1995;63:327–334. doi: 10.1016/0304-3959(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg W F, Mogil J S, Pilati M L, Boun C, Wong S K, Liebeskind J C. Proc West Pharmacol Soc. 1994;37:141–143. [PubMed] [Google Scholar]
- 7.Mogil J S, Richards S P, O'Toole L A, Helms M L, Mitchell S R, Kest B, Belknap J K. J Neurosci. 1997;17:7995–8002. doi: 10.1523/JNEUROSCI.17-20-07995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lander E S, Schork N J. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 9.Kavaliers M, Choleris E. Brain Res. 1997;768:30–36. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 10.Gear R W, Gordon N C, Heller P H, Paul S M, Miaskowski C, Levine J D. Neurosci Lett. 1996;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- 11.Gear R W, Miaskowski C, Gordon N C, Paul S M, Heller P H, Levine J D. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 12.Gear R W, Miaskowski C, Gordon N C, Paul S M, Heller P H, Levine J D. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 13.Gear R W, Miaskowski C, Gordon N C, Paul S M, Heller P H, Levine J D. J Pain. 2000;1:122–127. [Google Scholar]
- 14.Tallarida R J, Murray R B. Manual of Pharmacologic Calculation. New York: Springer; 1981. [Google Scholar]
- 15.Lander E S, Green P, Abrahamson J, Barlow A, Daly M J, Lincoln S E, Newburg L. Genomics. 1987;1:174–171. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 16.Al-Obeidi F, Hruby V J, Hadley M E, Sawyer T K, Castrucci A M. Int J Pept Protein Res. 1990;35:228–234. doi: 10.1111/j.1399-3011.1990.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 17.Laursen S E, Belknap J K. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 18.Price D D, Hu J W, Dubner R, Gracely R H. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 19.Moore P A, Duncan G H, Scott D S, Gregg J M, Ghia J N. Pain. 1979;6:375–382. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 20.Lander E S, Kruglyak L. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 21.Cone R D, Lu D, Koppula S, Vage D I, Klungland H, Boston B, Chen W, Orth D N, Pouton C, Kesterson R A. Rec Prog Horm Res. 1996;51:287–317. [PubMed] [Google Scholar]
- 22.Tatro J B. Neuroimmunomodulation. 1996;3:259–284. doi: 10.1159/000097281. [DOI] [PubMed] [Google Scholar]
- 23.Kolesnikov Y, Jain S, Wilson R, Pasternak G W. Eur J Pharmacol. 1996;310:141–143. doi: 10.1016/0014-2999(96)00520-1. [DOI] [PubMed] [Google Scholar]
- 24.Han G, Quillan J M, Carlson K, Sadee W, Hruby V J. J Med Chem. 2003;46:810–819. doi: 10.1021/jm020355o. [DOI] [PubMed] [Google Scholar]
- 25.Sturm R A, Box N F, Ramsay M. BioEssays. 1998;20:712–721. doi: 10.1002/(SICI)1521-1878(199809)20:9<712::AID-BIES4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Rees J L, Birch-Machin M, Flanagan N, Healy E, Phillips S, Todd C. Ann NY Acad Sci. 1999;885:134–142. doi: 10.1111/j.1749-6632.1999.tb08670.x. [DOI] [PubMed] [Google Scholar]
- 27.Scott M C, Wakamatsu K, Ito S, Kadekaro A L, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing V J, Abdel-Malek Z A. J Cell Sci. 2002;115:2349–2355. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- 28.Schioth H B, Phillips S R, Rudzish R, Birch-Machin M A, Wikberg J E S, Rees J L. Biochem Biophys Res Commun. 1999;260:488–491. doi: 10.1006/bbrc.1999.0935. [DOI] [PubMed] [Google Scholar]
- 29.Healy E, Jordan S A, Budd P S, Suffolk R, Rees J L, Jackson I J. Hum Mol Genet. 2001;10:2397–2402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau J H, Frankel W N. Nat Genet. 2000;25:381–384. doi: 10.1038/78051. [DOI] [PubMed] [Google Scholar]
- 31.Belknap J K, Hitzemann R, Crabbe J C, Phillips T J, Buck K J, Williams R W. Behav Genet. 2001;31:5–15. doi: 10.1023/a:1010249607128. [DOI] [PubMed] [Google Scholar]
- 32.Korstanje R, Paigen B. Nat Genet. 2002;31:235–236. doi: 10.1038/ng0702-235. [DOI] [PubMed] [Google Scholar]
- 33.Glazier A M, Nadeau J H, Aitman T J. Science. 2002;298:2345–2349. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- 34.Wikberg J E S. Eur J Pharmacol. 1999;375:295–310. doi: 10.1016/s0014-2999(99)00298-8. [DOI] [PubMed] [Google Scholar]
- 35.Xia Y, Wikberg J E S, Chhajlani V. NeuroReport. 1995;6:2193–2196. doi: 10.1097/00001756-199511000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Basbaum A I, Fields H L. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 37.Walker J M, Akil H, Watson S J. Science. 1980;210:1247–1249. doi: 10.1126/science.6254152. [DOI] [PubMed] [Google Scholar]
- 38.Ohkubo T, Shibata M, Takahashi H, Naruse S. Experientia. 1985;41:627–628. doi: 10.1007/BF02007691. [DOI] [PubMed] [Google Scholar]
- 39.Gispen W H, Buitellar J, Wiegant V M, Terenius L, De Wied D. Eur J Pharmacol. 1976;39:393–397. doi: 10.1016/0014-2999(76)90150-3. [DOI] [PubMed] [Google Scholar]
- 40.Manzanares J, Wagner E J, Moore K E, Lookingland K J. Life Sci. 1993;53:795–801. doi: 10.1016/0024-3205(93)90501-s. [DOI] [PubMed] [Google Scholar]
- 41.Quillan J M, Sadee W. Pharmacol Res. 1997;14:713–719. doi: 10.1023/a:1012185919153. [DOI] [PubMed] [Google Scholar]
- 42. Craft, R. M. (2003) Clin. J. Pain, in press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.