Abstract
Congestive heart failure results in cardiovascular dysfunction and diminished vascular nitric oxide (NO) production. We hypothesized that overexpression of endothelial NO synthase (eNOS) within the endothelium would reduce the extent of contractile dysfunction in a murine model of infarct-induced congestive heart failure. We generated transgenic (TG) mice overexpressing the human eNOS gene. The TG mice displayed significantly enhanced eNOS protein levels and eNOS activity levels (10- to 12-fold greater) in the aorta and the coronary vasculature. Non-TG (NTg) and eNOS TG mice were subjected to permanent left anterior descending coronary artery occlusion and then observed for 1 mo. We assessed cardiac function in vivo by using echocardiography and ultraminiature ventricular pressure catheters. Myocardial infarct size was similar between study groups (≈70% of the risk zone). Survival was increased by 43% in the eNOS TG mice compared with NTg (P < 0.05). Fractional shortening and cardiac output were also significantly (P < 0.05) greater in the eNOS TG than in NTg. Interestingly, pulmonary edema was evident only in NTg mice, and no evidence of pulmonary edema was observed in the eNOS TG mice. Thus, targeted overexpression of the eNOS gene within the vascular endothelium in mice attenuates both cardiac and pulmonary dysfunction and dramatically improves survival during severe congestive heart failure.
Cardiovascular disease adversely affects the lives of millions of people. Often precipitated by myocardial infarction, congestive heart failure is characterized by severe ventricular dysfunction, remodeling, pulmonary edema, and decreased exercise capacity. Despite its prevalence, many of the mechanisms associated with the development and pathophysiology of heart failure remain undetermined. One area holding particular promise is the role of nitric oxide (NO) in congestive heart failure.
NO is a diffusible highly reactive gas formed by three NO synthase (NOS) isoforms: NOS 1 (neuronal), NOS 2 (inducible), and NOS 3 (endothelial). NO is constitutively produced by the vascular endothelium and serves to promote vascular homeostasis (1). NO generated by NOS 3 maintains normal vascular tone (1, 2), regulates leukocyte–endothelial cell interactions (3), inhibits platelet aggregation (4), limits smooth muscle cell proliferation (5), and may affect cardiac myocyte function (6). Given this favorable physiological profile, it is likely that NO therapy may prove beneficial in a number of disease states. Recently, inhalation of exogenous NO gas has been shown to be a highly effective therapy for infant respiratory distress syndrome in humans (7). Since its identification as endothelium-derived relaxing factor in 1980 (8), NO has been implicated in numerous pathologic sequelae as both a beneficial and a deleterious moiety.
Several studies have provided evidence that NO production by endothelial NOS (eNOS) is markedly diminished in congestive heart failure (9–14). Patients with heart failure have diminished functional eNOS capacity compared with normal humans both at rest and during submaximal exercise (9). It was also shown that serum collected from heart failure patients causes endothelial cell dysfunction by down-regulating eNOS activity (10). Wiemer et al. (11) reported decreased NO bioavailability in a rodent model of infarct-induced heart failure and found this decrement in NO availability in both normotensive and hypertensive rats after acute myocardial infarction.
We are unaware of any previous studies of congestive heart failure in any eNOS overexpressing mouse line. Such studies are important for two reasons. First, endothelial overexpression of eNOS would theoretically result in a relatively low site-specific (physiological) level of NO production. Overexpression of eNOS may mimic the clinical observations of pharmacologic agents (e.g., statins and angiotensin-converting enzyme inhibitors) inducing the production of NO resulting in cardioprotection. Ultimately, this would clarify whether moderate elevations in NO production confined to the vascular endothelium affect the pathogenesis of congestive heart failure. In the present study, we sought to determine whether genetic overexpression of eNOS in the vascular endothelium could affect the severity of congestive heart failure after the acute myocardial infarction model in mice.
Methods
Transgenic (TG) Mice.
A DNA fragment containing the human eNOS gene was isolated from a homemade human genomic cosmid library (15–17) by using eNOS cDNA (kindly donated by S. Janssens, Univ. of Leuven, Leuven, Belgium) (18) as a probe. In addition, the DNA fragment contained ≈6 kb of 5′ natural flanking sequence, including the native eNOS promoter, and ≈3 kb of 3′ sequence to the gene. Thus, promoter DNA elements were included that have been shown to be essential in transcriptional regulation as well as elements responsible for the tissue distribution of eNOS expression (16, 19). Also, the endothelium enhancer element that is located 4.9 kb upstream from the transcription start site of the eNOS gene (16) is included in this construct. Vector sequences were removed by restriction endonucleases. A solution of 1–2 μg/ml DNA was used for microinjection of fertilized oocytes from FVB donor mice and transplanted into the oviducts of pseudopregnant B10×CBA mice. Founder mice and offspring were genotyped by PCR on DNA isolated from tail biopsies. Primers used were 5′-GTCCTGCAGACCGTGCAGC-3′ (sense) and 5′-GGCTGTTGGTGTCTGAGCCG-3′ (antisense). Mice were backcrossed to C57BL/6 for at least seven generations (≈99% C57BL/6). TG mice were originally generated in Rotterdam and have been extensively characterized previously (16).
An equal number of male and female mice (3–4 mo old) were used in the NTg and TG groups in the present study. The individual performing all experiments was blinded to the mouse genotype until all data were fully analyzed. All experiments performed at Louisiana State University Health Sciences Center conformed to state and federal regulations regarding animal experimentation and to American Physiological Society Guidelines.
Western Blotting and Immunohistochemistry.
Aortas were freshly collected and homogenized in 50 mM Tris⋅HCl, pH 7.4, containing 1 mM EDTA, 0.25 M sucrose, and 20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. Western blotting was performed as described previously (20). Twenty-five micrograms of protein (BCA protein assay kit, Pierce) was applied to each lane. Anti-eNOS was obtained from Santa Cruz Biotechnology. This antibody was also used for immunohistochemistry experiments, which were performed according to Bakker et al. (21).
eNOS Activity Assay.
Aortas were freshly collected and homogenized in 50 mM Tris⋅HCl, pH 7.4, containing 1 mM EDTA, 0.25 M sucrose, and 20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate. eNOS activity assays were performed by measuring l-arginine to l-citrulline conversion with a NOS assay kit (Calbiochem) according to the manufacturer's instructions. In additional experiments, eNOS enzymatic activity was also measured in freshly collected hearts. For myocardial eNOS activity, a membrane-enriched fraction was used instead of a tissue homogenate. All measurements of eNOS activity were normalized for total protein content. Protein content was measured by using the BCA protein assay kit (Pierce).
Myocardial Infarction Protocol.
Ligation of the left anterior descending coronary artery (LAD) was performed similar to methods described (22). Briefly, mice were fully anesthetized with i.p. injections of ketamine (50 mg/kg) and pentobarbital sodium (50 mg/kg). The animals were then attached to a surgical board with their ventral side up. A median sternotomy was performed, and the proximal LAD was visualized and completely ligated by passing a 7–0 silk suture mounted on a tapered needle (BV-1, Ethicon, Somerville, NJ) completely around the artery and fully ligating the LAD. The occlusion remained intact throughout the 4-wk protocol.
In additional experiments (n = 6 per group), both NTg and eNOS TG mice were subjected to permanent LAD ligation for 24 h to determine the extent of acute myocardial infarction. Mice were allowed to fully recover after LAD ligation and were reanesthetized at 24 h after LAD occlusion. Myocardial area-at-risk and myocardial infarct size per area at risk and left ventricle (LV) were determined in each animal as described (23).
Evaluation of Arterial and Left Ventricular Hemodynamics.
A 1.4 French Millar Instruments (SPR-671; Houston) pressure transduction catheter was inserted similar to methods described (22, 23). Data for each animal were calculated from at least 10 sec of chart recording (arithmetic mean of at least 50 cardiac cycles).
Echocardiographic Assessment of Left Ventricular Function.
Transthoracic echocardiography of the LV using a 15-MHz linear array transducer (15L8) interfaced with a Sequoia C256 (Acuson, Mountain View, CA) was performed as described (22). All data were calculated from 10 cardiac cycles per experiment.
Pulmonary Edema.
Accumulation of pulmonary fluid was assessed by weighing the (wet) lungs from mice subjected to myocardial infarction (or sham). The lungs were then placed in a drying oven (Econotherm Laboratory Oven, Precision Systems, Natick, MA) for 7 d at 40°C. The lungs were weighed and the dry weights recorded. The arithmetic difference between the wet and dry weights yielded the pulmonary fluid accumulation values.
Statistical Analyses.
Data were analyzed by ANOVA with post hoc analysis by using statview (SAS, Cary, NC) software. Data are reported as means ± standard error of the mean with differences accepted as significant when P ≤ 0.05.
Results
Characterization of eNOS TG Mice.
TG overexpression of eNOS resulted in significant increases in eNOS protein levels according to Western blot analysis (Fig. 1A) and immunohistochemistry (Fig. 1B). This increase in protein levels was also reflected by an increase in eNOS activity (Fig. 2). In addition, we observed increased eNOS protein levels in the myocardium of eNOS TG mice by using Western blotting (Fig. 3A) and immunostaining techniques (Fig. 3B).
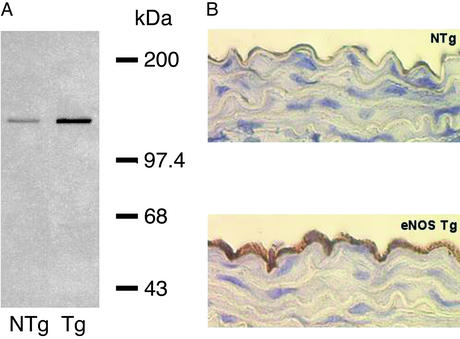
Figure 1.
(A) Representative Western blot from aorta of NTg and eNOS TG mice. (B) Representative photomicrograph of eNOS immunohistochemistry demonstrating increased abundance of eNOS protein in the endothelium of eNOS TG mice compared with NTg.
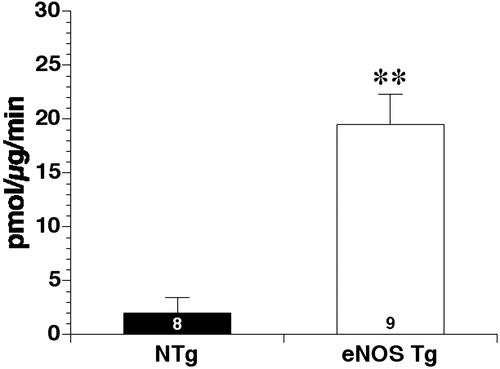
Figure 2.
Assessment of aortic eNOS enzymatic activity in eight NTg and nine eNOS TG mice. eNOS activity was determined by measuring the conversion of l-arginine to l-citrulline (pmol/μg/min). TG mice displayed significantly (**, P < 0.01) enhanced eNOS enzyme activity compared with NTg control mice.
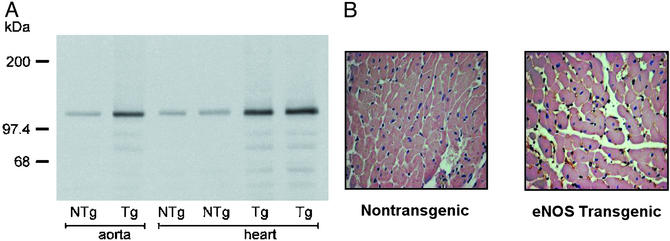
Figure 3.
(A) Aortic and myocardial eNOS protein levels as measured by Western blot analysis in NTg and eNOS TG mice. eNOS protein expression is significantly enhanced in the aorta and myocardium of eNOS TG mice. (B) Representative immunohistochemical staining of myocardial tissue harvested from NTg and eNOS TG mice demonstrating increased eNOS protein in the myocardium of eNOS TG mice compared with the NTg control mice.
Severity of Myocardial Infarction in eNOS TG Mice.
The extent of myocardial infarction was measured in NTg and eNOS TG mice at 24 h after permanent LAD coronary artery occlusion (Fig. 4). We observed no differences in the area at risk per LV or in the infarct size per area at risk or infarct size per LV. Thus, the severity of acute myocardial infarction was similar in the NTg and eNOS TG before the onset of congestive heart failure.
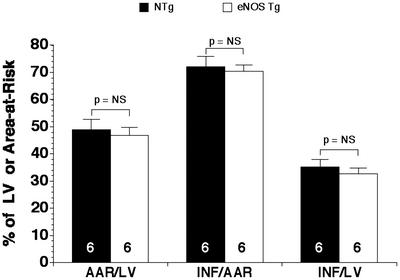
Figure 4.
Myocardial infarct size in NTg and eNOS TG mice subjected to permanent myocardial occlusion. The area-at-risk (AAR) per LV was similar (P = NS) in the NTg and eNOS TG groups. In addition, the infarct size (INF) per AAR and LV was similar in the NTg and eNOS TG groups (P = NS). n = 6 per group.
Effect of eNOS Overexpression on Ventricular Dimensions and Function.
Overexpression of eNOS did not significantly affect cardiac function or LV dimensions in control mice under baseline conditions (Table 1). Specifically, LV diameters, wall thicknesses, and fractional shortening were similar between the NTg and eNOS TG groups [P = not significant (NS)].
Table 1.
In vivo left ventricular dimensions of NTg and eNOS TG mice not subjected to any surgery
| LVFS, % | LV chamber
|
LV anterior wall
|
LV posterior wall
|
||||
|---|---|---|---|---|---|---|---|
| Diastole, mm | Systole, mm | Diastole, mm | Systole, mm | Diastole, mm | Systole, mm | ||
| NTg | 25.0 ± 1.1 | 3.55 ± 0.18 | 2.68 ± 0.16 | 0.87 ± 0.03 | 1.15 ± 0.03 | 0.95 ± 0.05 | 1.12 ± 0.05 |
| eNOSTG | 26.4 ± 2.1 | 3.53 ± 0.15 | 2.63 ± 0.17 | 0.88 ± 0.03 | 1.20 ± 0.03 | 0.89 ± 0.06 | 1.13 ± 0.06 |
There were no significant differences between the two groups in any of the parameters shown. LVFS, left ventricular fractional shortening; n = 10 per group.
Survival During Congestive Heart Failure.
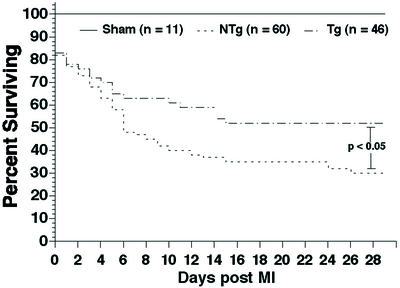
eNOS TG and NTg mice experienced a significant (P < 0.01) degree of mortality compared with sham-operated mice during the congestive heart failure protocol (Fig. 5). However, overexpression of eNOS significantly (P < 0.05) improved survival compared with NTg mice. Survival was increased by ≈43% in the eNOS TG group at 1 mo after myocardial infarction.
Figure 5.
Kaplan–Meier survival curve in sham NTg, NTg, and eNOS TG mice during the 4 wk after myocardial infarction. Both NTg and eNOS TG exhibited significant (P < 0.01) decreases in survival compared with sham mice after 1 mo of myocardial infarction. eNOS TG mice demonstrated significantly (P < 0.05) greater survival compared with NTg during the congestive heart failure protocol.
Hemodynamics and eNOS Overexpression During Congestive Heart Failure.
In NTg and eNOS TG mice subjected to myocardial infarction, there were no significant differences in systemic hemodynamics measured at 4 wk after myocardial infarctionγ compared with sham (Table 2). Although LV systolic pressure was not significantly different among the three experimental groups, LV end diastolic pressure was significantly (P < 0.05) higher in the eNOS TG and NTg hearts compared with sham. In addition, LV developed pressure was significantly (P < 0.05) lower in the NTg and eNOS TG hearts compared with sham.
Table 2.
Systemic hemodynamics, ventricular hemodynamics, and cardiac hypertrophy in sham, NTg, and eNOS TG mice subjected to 1 mo of coronary artery occlusion
| HR, bpm | MABP, mmHg | SBP, mmHg | DBP, mmHg | LVSP, mmHg | LVEDP, mmHg | HW/BW, mg/g | |
|---|---|---|---|---|---|---|---|
| Sham | 393 ± 12 | 73 ± 4 | 86 ± 4 | 60 ± 4 | 88 ± 3 | 1 ± 1 | 4.43 ± 0.15 |
| NTg | 403 ± 10 | 72 ± 3 | 85 ± 3 | 61 ± 3 | 84 ± 4 | 10 ± 4* | 6.25 ± 0.24** |
| eNOSTG | 422 ± 12 | 66 ± 5 | 78 ± 5 | 56 ± 4 | 81 ± 5 | 7 ± 5* | 6.46 ± 0.25** |
HR, heart rate; MABP, mean arterial blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVSP, left ventricular systolic pressure; LVEDP, LV end diastolic pressure; HW/BW, heart weight to body weight ratio.
P < 0.05 vs. sham;
P < 0.01 vs. sham; otherwise P = NS; n = 10–12/group.
Cardiac Hypertrophy.
Both NTg and eNOS TG hearts demonstrated significant (P < 0.01) increases in heart-to-body-weight ratios 1 mo after coronary artery occlusion when compared with sham (Table 2). The extent of cardiac hypertrophy was not significantly different between the NTg and eNOS TG groups of hearts.
Left Ventricular Dimensions.
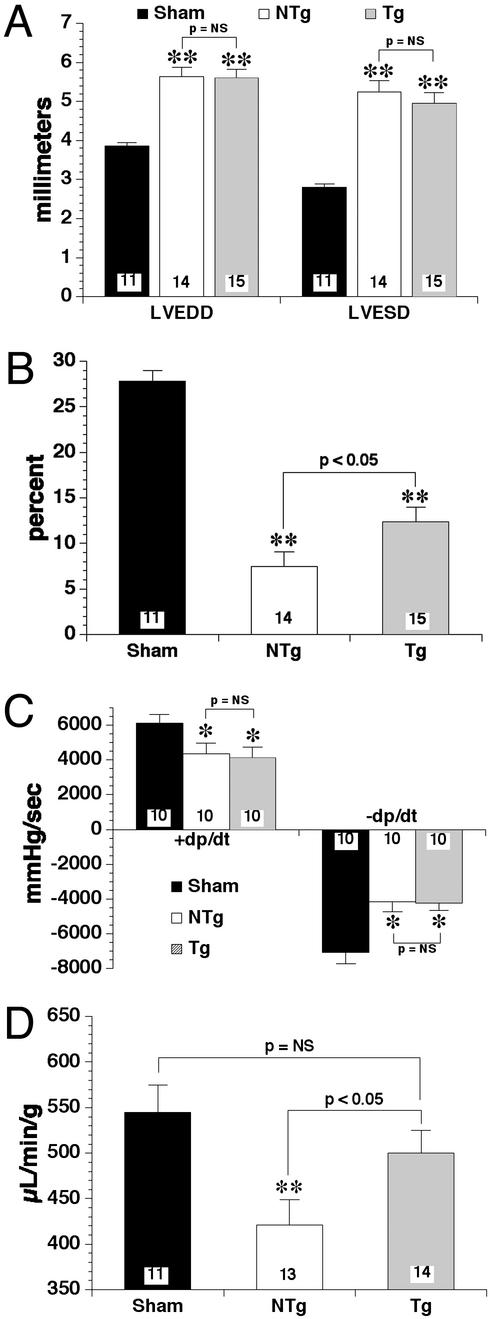
In addition to hypertrophy, the present model of congestive heart failure induces profound effects on ventricular dimensions (Fig. 6A). Namely, left ventricular chamber diameters are significantly (P < 0.01) greater in diastole and systole in the NTg (5.64 ± 0.24 mm; 5.24 ± 0.29 mm) and eNOS TG (5.60 ± 0.22 mm; 4.95 ± 0.27 mm) hearts compared with sham (3.86 ± 0.09 mm; 2.80 ± 0.09 mm). The differences between the NTg and eNOS TG mice were not statistically significant (P = NS). Fractional shortening (Fig. 6B) was also significantly (P < 0.01) lower in both heart failure groups compared with sham (27.8 ± 1.2%). More importantly, eNOS TG (12.4 ± 1.6%) hearts exhibited a significantly (P < 0.05) greater extent of fractional shortening compared with NTg (7.5 ± 1.6%).
Figure 6.
(A) LV chamber diameters during diastole (LVEDD) and systole (LVESD) in sham NTg, NTg, and eNOS TG mice after 4 wk of coronary artery occlusion. NTg and eNOS TG mice exhibited significant (**, P < 0.01) LV chamber dilatation in diastole and systole compared with sham-operated NTg mice. There were no significant differences in LV diameters between NTg and eNOS TG after 4 wk of heart failure. (B) Fractional shortening (%) was significantly attenuated in both groups (NTg and eNOS TG) of mice subjected to coronary artery occlusion compared with sham (**, P < 0.01). However, fractional shortening was significantly (P < 0.05) higher in the eNOS TG group compared with NTg. (C) Left ventricular maximum (+) and minimum (−) dP/dt was assessed by using pressure transduction catheters. Myocardial infarction induced significant (*, P < 0.05) depression of dP/dt in NTg and eNOS TG mice compared with sham. However, dP/dt was not significantly different between NTg and eNOS TG (P = NS). (D) By using transthoracic Doppler-assisted echocardiography, cardiac output was determined in sham NTg, NTg, and eNOS TG mice after 1 mo of coronary artery occlusion. Cardiac output was significantly (P < 0.01) reduced in the NTg compared with sham NTg. eNOS TG mice did not exhibit a significant decrement in cardiac output compared with sham. Overexpression of eNOS (TG) resulted in a significantly (P < 0.05) higher cardiac output compared with NTg mice after the 4-wk congestive heart failure protocol. Numbers of mice are located within the bars.
Indices of Cardiac Function.
At 4 wk of coronary artery occlusion, sham NTg, NTg, and eNOS TG mice were subjected to in vivo left ventricular catheterization for determination of intracyclic ventricular pressures (Fig. 6C). NTg [4,336 ± 623 mmHg/sec (1 mmHg = 133 Pa)] and eNOS TG (4,112 ± 611 mmHg/sec) exhibited significantly (P < 0.05) diminished maximum (+) dP/dt compared with sham (6,109 ± 491 mmHg/sec). Similarly, minimum (−) dP/dt was significantly attenuated in NTg (−4,145 ± −578 mmHg/sec) and eNOS TG (−4,225 ± −425 mmHg/sec) hearts compared with sham (−7,055 ± −662 mmHg/sec). There were no significant differences between NTg and eNOS TG in maximum or minimum dP/dt.
By using Doppler flow analysis during echocardiography, cardiac output was measured in all groups of mice after 4 wk of myocardial infarction (Fig. 6D). Cardiac output only in NTg (421 ± 28 μl/min/g) mice was significantly (P < 0.01) reduced compared with sham (545 ± 30 μl/min/g). However, cardiac output was not significantly (P = NS) diminished in eNOS TG (500 ± 25 μl/min/g) mice compared with sham. Cardiac output in eNOS TG was also significantly (P < 0.05) higher than NTg.
Pulmonary Edema.
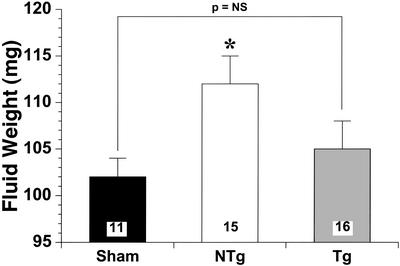
Pulmonary edema is associated with depressed cardiac function in the setting of infarct-induced LV failure. Pulmonary edema (Fig. 7) is expressed as the absolute fluid weight in the lungs of sham NTg, NTg, and eNOS TG after 4 wk of myocardial infarction. Sham (102 ± 2 mg) fluid weight was not significantly different from eNOS TG (105 ± 3 mg). However, NTg (112 ± 3 mg) pulmonary fluid accumulation was significantly (P < 0.05) higher than sham NTg mice.
Figure 7.
Pulmonary edema was assessed by net fluid weights in 11 sham, 15 NTg, and 16 eNOS TG mouse lungs. NTg mice exhibited significantly (P < 0.05) greater fluid accumulation compared with sham lungs. However, eNOS TG mice did not demonstrate any significant differences compared with NTg.
Discussion
We provide compelling evidence for a beneficial role of enhanced eNOS-derived NO in congestive heart failure. Improvement in survival of congestive heart failure in the eNOS TG group is the paramount finding of the present study. In addition to improvements in mortality, mice overexpressing eNOS by 10- to 12-fold exhibited reductions in pulmonary edema and improvement in cardiac function. All of these improvements occurred in the eNOS TG mice without apparent differences in baseline ventricular morphology and function. However, eNOS overexpression did not attenuate the extent of cardiac hypertrophy or improve cardiac contractility. It is likely that the improvements in cardiac function and survival are a result of a decrease in vascular resistance in the eNOS TG mice. These findings support the idea that physiological levels of NO exert beneficial effects in the setting of congestive heart failure.
Before the discovery that the endothelial-derived relaxing factor was NO, it was not appreciated that the primary mechanism of action of traditional nitrates was via the release of NO and subsequent vasodilation. Nitrovasodilators have been used since at least the mid-19th century (24) for the treatment of angina pectoris. More recently, nitrovasodilators have been used for the treatment of heart failure (25–27). Nitrovasodilators have also been shown to improve exercise tolerance in patients with severe congestive heart failure (25, 26). It is now known that the nitrovasodilators' mechanism of action is via the release of NO from the parent compound. Interestingly, administration of inhaled NO to congestive heart failure patients exerts beneficial effects without significantly altering systemic hemodynamics (28, 29). Although systemic hemodynamics were unchanged, the mechanism still appeared to be related to alterations in pulmonary blood flow (i.e., decreased pulmonary vascular resistance). In the present study, it is possible that overexpression of eNOS exerted beneficial regional effects on pulmonary blood flow.
Endothelial dysfunction occurs in patients with heart failure (30–33). Experimental studies (11–14) indicate that a decrease in NO bioavailability is associated with heart failure and actually exerts deleterious effects during heart failure. eNOS-deficient mice have been subjected to coronary artery ligation and heart failure (34). This study revealed the important role of endogenous NO in attenuating the pathophysiology associated with congestive heart failure (34). Scherrer-Crosbie et al. (34) found that end diastolic diameter and end diastolic volume were increased, whereas fractional shortening, contractility, and survival were depressed in eNOS-deficient mice compared with wild-type after 4 wk of LAD ligation. Furthermore, LV hypertrophy and myocyte width were significantly increased in the eNOS-deficient mice. Interestingly, capillary density was lower in the post-myocardial infarction eNOS deficient mice than wild type, implying a role for NO in postinfarction capillary preservation. Scherrer-Crosbie et al. (34, 35) also demonstrated that genetic deficiency of eNOS resulted in significant pulmonary hypertension and right ventricular remodeling. In the present study, eNOS TG mice may have improved coronary vascularity compared with NTg mice during congestive heart failure. These findings, in conjunction with the present results, provide significant support for a protective role of eNOS-derived NO in infarct-induced congestive heart failure.
Several lines of eNOS TG mice have been developed that overexpress eNOS in endothelial cells under the direction of a preproendothelin promoter (36) and cardiac myocytes controlled by an α-MHC promoter (37). Although no specific cardiac phenotype has yet been reported at baseline in the endothelial eNOS TG mouse (36), Brunner et al. (37) found mild negative effects in terms of cardiac-inducible calcium sensitivity. Although calcium sensitivity was not the focus of the present study, dobutamine-stress data indirectly indicate no difference between the eNOS TG and NTg hearts in terms of calcium sensitivity. It is likely that this differential effect is related to the higher abundance of eNOS protein in the Brunner et al. study (37) compared with the present findings. Additionally, the site of eNOS overexpression may be of critical importance.
NO has recently been suggested to contribute to the pathogenesis of heart failure (38–40), a tenet that is not uniformly accepted (40). The current study does not support the idea of NO as a deleterious mediator of congestive heart failure. Although not the focus of our study per se, it is possible that overproduction of NO via iNOS actually causes negative effects during heart failure due to the high capacity of iNOS to produce NO. More studies of iNOS and eNOS in congestive heart failure are required to fully elucidate this complex physiological system.
The present data demonstrate significant beneficial effects in eNOS TG mice during heart failure. Although the mechanism of enhanced survival appears to be related to improvement in ventricular function and/or abrogation of pulmonary edema, the mechanism of improved ventricular function is unclear. One possible mechanism is enhanced blood flow to the compensating, surviving myocardium in the eNOS TG mice during heart failure. This effect may occur by blunting the vasoconstrictor response (angiotensin II, endothelin, etc.) during heart failure. The increased bioavailability of NO may attenuate the loss of capillaries that occurs during heart failure and may actually potentiate the formation of new vessels. In addition, the enhanced NO production in the eNOS TG mice may serve as an oxidant scavenger during heart failure, thereby minimizing the deleterious effects of superoxide and other reactive oxygen species. A very likely explanation for the significant improvement in cardiac function and survival in the eNOS TG mice is related to a reduction in systemic vascular resistance. Systemic overexpression of the eNOS enzyme within the vascular endothelium may simply reduce cardiac afterload, and this mechanism may be responsible for the increase in cardiac output, attenuation of pulmonary edema, and increased survival. Thus, eNOS overexpression does not directly improve left ventricular function in the setting of congestive heart failure. Ultimately, additional studies will be required to elucidate the multitude of mechanistic possibilities and their relative contributions to the in vivo pathophysiology of congestive heart failure.
In summary, overexpression of the eNOS enzyme significantly improves survival and attenuates the extent of congestive heart failure after severe myocardial infarction in mice. These data provided clear evidence of the beneficial role of enhanced eNOS-derived NO production during congestive heart failure. Future studies should be directed toward identifying the mechanism of this protective effect. In the future, therapies might be developed to improve vascular eNOS function as a means to improve clinical outcomes in patients with congestive heart failure.
Acknowledgments
We acknowledge the expert technical assistance of Michael B. Hicks during the course of these studies. This work was funded by National Institutes of Health Grants RO1 HL-60849 and PO1 DK-43785 (to D.J.L.).
Abbreviations
- NOS
NO synthase
- eNOS
endothelial NOS
- LAD
left anterior descending coronary artery
- TG
transgenic
- NTg
nontransgenic
- LV
left ventricle
- NS
not significant
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ignarro L J, Napoli C, Loscalzo Circ Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 2.Gruetter C A, Barry B K, McNamara D B, Gruetter D Y, Kadowitz P J, Ignarro L. J Cyclic Nucleotide Res. 1979;5:211–214. [PubMed] [Google Scholar]
- 3.Kubes P, Suzuki N, Granger D N. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellion B T, Ignarro L J, Ohlstein E H, Pontecorvo E G, Hyman A L, Kadowitz P J. Blood. 1981;57:946–955. [PubMed] [Google Scholar]
- 5.Ignarro L J, Buga G M, Wei L H, Bauer P H, Wu G, del Soldato P. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly R A, Balligand J L, Smith T W. Circ Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- 7.Roberts J J, Fineman J R, Morin F C, III, Shaul P W, Rimar S, Schreiber M D, Polia R A, Zwass M S, Zayek M M, Gross I, et al. N Engl J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 8.Furchgott R F, Zawadzki J V. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 9.Katz S D, Khan T, Zeballos G A, Mathew L, Potharlanka P, Knecht M, Whelan J. Circulation. 1999;99:2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 10.Agnoletti L, Curello S, Bachetti T, Malacarne F, Gaia G, Comini L, Volterrani M, Bonetti P, Parrinello G, Cadei M, et al. Circulation. 1999;100:1983–1991. doi: 10.1161/01.cir.100.19.1983. [DOI] [PubMed] [Google Scholar]
- 11.Wiemer G, Itter G, Malinski T, Linz W. Hypertension. 2001;38:1367–1371. doi: 10.1161/hy1101.096115. [DOI] [PubMed] [Google Scholar]
- 12.Arimura K, Egashira K, Nakamura R, Ide T, Tsutsui H, Shimokawa H, Takeshita A. Am J Physiol. 2001;280:H68–H75. doi: 10.1152/ajpheart.2001.280.1.H68. [DOI] [PubMed] [Google Scholar]
- 13.Sun D, Huang A, Zhao G, Bernstein R, Forfia P, Xu X, Koller A, Kaley G, Hintze T H. Am J Physiol. 2000;278:H461–H468. doi: 10.1152/ajpheart.2000.278.2.H461. [DOI] [PubMed] [Google Scholar]
- 14.Recchia F A, McConnell P I, Bernstein R D, Vogel T R, Xu X, Hintze T H. Circ Res. 1998;83:969–979. doi: 10.1161/01.res.83.10.969. [DOI] [PubMed] [Google Scholar]
- 15.van Haperen R, van Tol A, Vermeulen P, Jauhiainen M, van Gent T, van den Berg P, Ehnholm S, Grosveld F, van der Kamp A, de Crom R. Arterioscler Thromb Vasc Biol. 2000;20:1082–1088. doi: 10.1161/01.atv.20.4.1082. [DOI] [PubMed] [Google Scholar]
- 16. van Haperen, R., de Waard, M., van Deel, E., Mees, B., Kutryk, M., van Aken, K., Hamming, J., Grosveld, F., Duncker, D. J. & de Crom, R. (2003) J. Biol. Chem., in press. [DOI] [PubMed]
- 17.Janssens S P, Shimouchi A, Quertermous T, Bloch D B, Bloch K D. J Biol Chem. 1992;267:14519–14522. [PubMed] [Google Scholar]
- 18.Govers R, Rabelink T J. Am J Physiol. 2001;280:F193–F206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 19.Laumonnier Y, Nadaud S, Agrapart M, Soubrier F. J Biol Chem. 2000;275:40732–40741. doi: 10.1074/jbc.M004696200. [DOI] [PubMed] [Google Scholar]
- 20.de Crom R, van Haperen R, Janssens R, Visser P, Willemsen R, Grosveld F, van der Kamp A. Biochim Biophys Acta. 1999;1437:378–392. doi: 10.1016/s1388-1981(99)00017-7. [DOI] [PubMed] [Google Scholar]
- 21.Bakker C, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen A T, Oostra B A, Willemsen R. Exp Cell Res. 2000;258:162–170. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- 22.Jones S P, Trocha S D, Lefer D J. Arterioscler Thromb Vasc Biol. 2001;21:2059–2064. doi: 10.1161/hq1201.099509. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz J N, Robbins J. Am J Physiol. 1997;272:H1137–H1146. doi: 10.1152/ajpheart.1997.272.3.H1137. [DOI] [PubMed] [Google Scholar]
- 24.Brunton T L. Lancet. 1867;2:97–98. [Google Scholar]
- 25.Guiha N H, Cohn J N, Mikulic E, Franciosa J A, Limas C J. N Engl J Med. 1974;291:587–592. doi: 10.1056/NEJM197409192911201. [DOI] [PubMed] [Google Scholar]
- 26.Miller R R, Vismara L A, Zelis R, Amsterdam E A, Mason D T. Circulation. 1975;51:328–336. doi: 10.1161/01.cir.51.2.328. [DOI] [PubMed] [Google Scholar]
- 27.Franciosa J A, Nordstrom L A, Cohn J N. J Am Med Assoc. 1978;240:443–446. [PubMed] [Google Scholar]
- 28.Matsumoto A, Momomura S, Hirata Y, Aoyagi T, Sugiura S, Omata M. Lancet. 1997;349:999–1000. doi: 10.1016/s0140-6736(05)62897-8. [DOI] [PubMed] [Google Scholar]
- 29.Koelling T M, Kirmse M, Di Salvo T G, Dee G W, Zapol W M, Semigren M J. Am J Cardiol. 1998;81:1494–1497. doi: 10.1016/s0002-9149(98)00214-8. [DOI] [PubMed] [Google Scholar]
- 30.Drexler H, Hayoz D, Munzel T, Hornig B, Just H, Brunner H R, Zelis R. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 31.Vanhoutte P M. J Mol Cell Cardiol. 1996;28:2233–2240. doi: 10.1006/jmcc.1996.0215. [DOI] [PubMed] [Google Scholar]
- 32.Smith C J, Sun D, Hoegler C, Roth B S, Zhang X, Zhao G, Xu X B, Kobari Y, Pritchard K, Jr, Sessa W C, Hintze T H. Circ Res. 1996;78:58–64. doi: 10.1161/01.res.78.1.58. [DOI] [PubMed] [Google Scholar]
- 33.Comini L, Bachetti T, Gaia G, Pasini E, Agnoletti L, Pepi P, Ceconi C, Curello S, Ferrari R. J Mol Cell Cardiol. 1996;28:2241–2248. doi: 10.1006/jmcc.1996.0216. [DOI] [PubMed] [Google Scholar]
- 34.Scherrer-Crosbie M, Ullrich R, Bloch K D, Nakajima H, Nasseri B, Aretz T, Lindsey M L, Vancon A C, Huang P L, Lee R T, et al. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 35.Steudel W, Scherrer-Crosbie M, Bloch K D, Weimann J, Huang P L, Jones R C, Picard M H, Zapol W M. J Clin Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Circulation. 2001;104:3097–3102. doi: 10.1161/hc5001.101966. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer D B, Colucci W S. Cardiol Clin. 1998;16:657–664. doi: 10.1016/s0733-8651(05)70042-4. [DOI] [PubMed] [Google Scholar]
- 39.Drexler H. Circulation. 1999;99:2972–2975. doi: 10.1161/01.cir.99.23.2972. [DOI] [PubMed] [Google Scholar]
- 40.Paulus W J, Frantz S, Kelly R A. Circulation. 2001;104:2260–2262. [PubMed] [Google Scholar]