Abstract
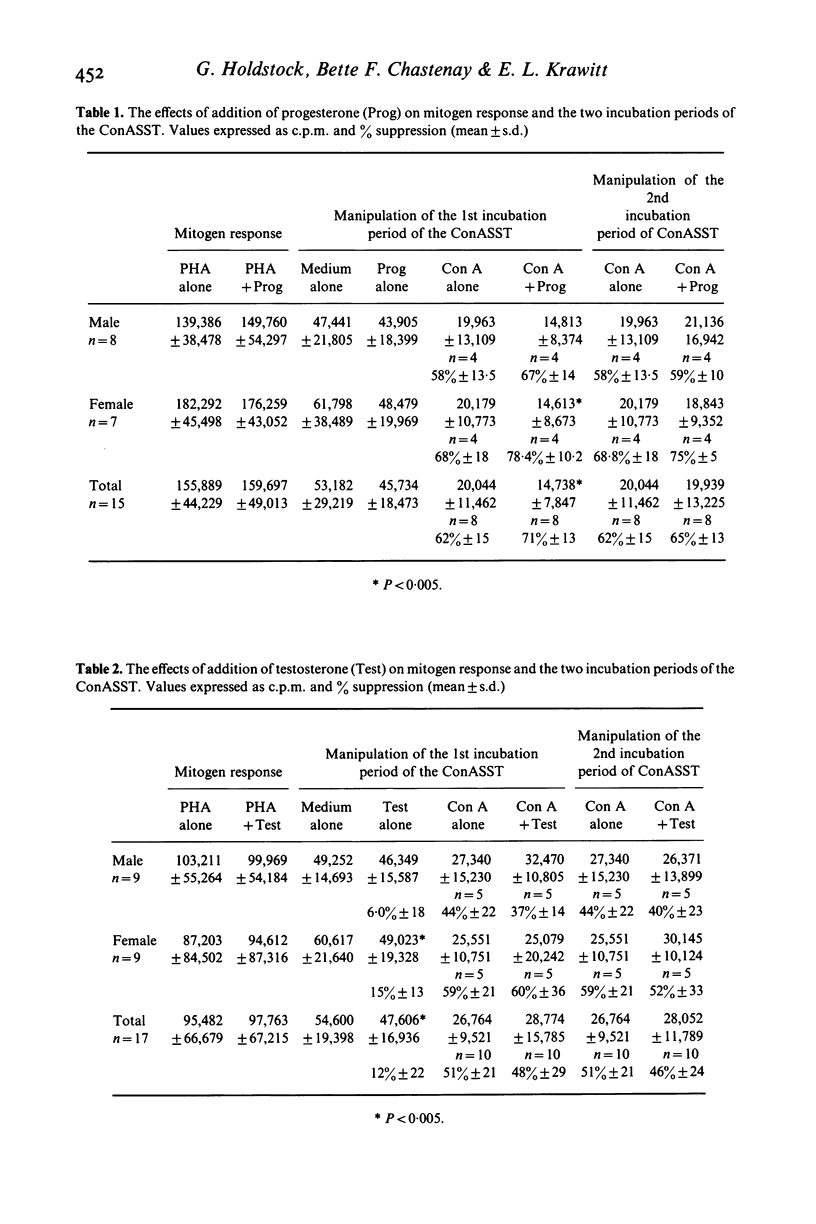
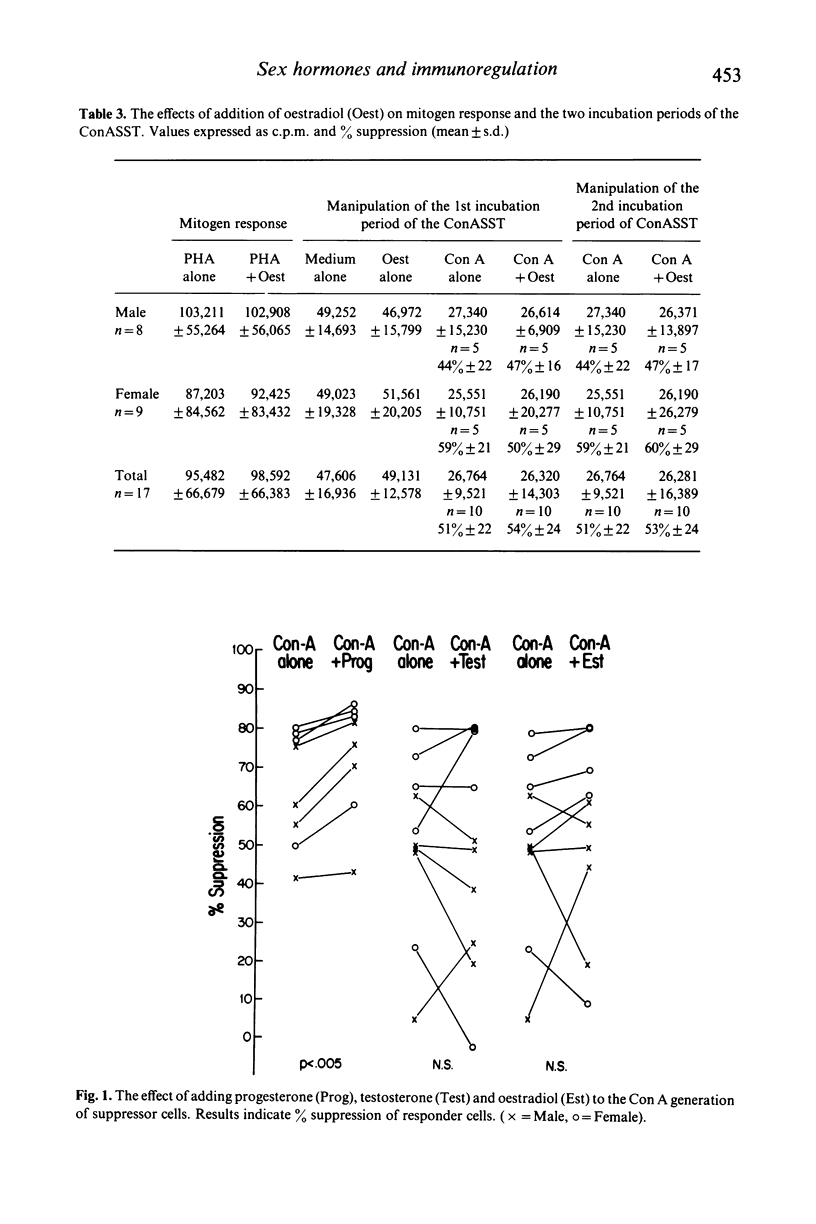
Clinical observations on differences in the sexual incidence of diseases associated with defects of immune regulation, and of the occasional beneficial effects of pregnancy on disease course suggest that endocrine mechanisms may be important in the immuno-pathogenesis of these disorders. To investigate this possibility the in vitro effects of testosterone, oestradiol and progesterone on selected aspects of immune regulation were studied in normal adults. We observed the effects of these hormones on a mitogen-induced suppressor T-cell system and a monocyte mediated prostaglandin-producing suppressor cell system. The addition of progesterone but not oestradiol or testosterone to the Concanavalin A (Con A) generation of T lymphocyte suppressor cells produced significantly increased suppressor cell activity (P less than 0.005). Pre-incubation of lymphocytes with testosterone but not oestradiol or progesterone in the absence in the absence of Con A resulted in the generation of modest, but highly significant suppressor cell activity (P less than 0.005) No effect on the prostaglandin-producing suppressor cell activity was observed. These findings suggest that certain endocrine changes may alter immunoregulatory function and account for some of the clinical observations previously noted in diseases associated with defects of immune regulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bole G. G., Jr, Friedlaender M. H., Smith C. K. Rheumatic symptoms and serological abnormalities induced by oral contraceptives. Lancet. 1969 Feb 15;1(7590):323–326. doi: 10.1016/s0140-6736(69)91294-x. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Garrett T. J. Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med. 1972 Nov 1;136(5):1098–1116. doi: 10.1084/jem.136.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARSENSTEIN M., POLLAK V. E., KARK R. M. Systemic lupus erythematosus and pregnancy. N Engl J Med. 1962 Jul 26;267:165–169. doi: 10.1056/NEJM196207262670401. [DOI] [PubMed] [Google Scholar]
- Gluud C., Tage-Jensen U., Bahnsen M., Dietrichson O., Svejgaard A. Autoantibodies, histocompatibility antigens and testosterone in males with alcoholic liver cirrhosis. Clin Exp Immunol. 1981 Apr;44(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper B., Whittingham S., Mathews J. D., Mackay I. R., Curnow D. H. Autoimmunity in a rural community. Clin Exp Immunol. 1972 Sep;12(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- Karpovitch X. L., Rosenkovitch E., Ben-Basset H., Izak G. Structure and functional alterations in lymphocytes induced by cryopreservation. Cryobiology. 1980 Feb;17(1):12–17. doi: 10.1016/0011-2240(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Knapp W., Posch B. Concanavalin A-induced suppressor cell activity opposing effects of hydrocortisone. J Immunol. 1980 Jan;124(1):168–172. [PubMed] [Google Scholar]
- London W. T., Drew J. S. Sex differences in response to hepatitis B infection among patients receiving chronic dialysis treatment. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2561–2563. doi: 10.1073/pnas.74.6.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels R. M., Rogers K. D. A sex difference in immunologic responsiveness. Pediatrics. 1971 Jan;47(1):120–123. [PubMed] [Google Scholar]
- Miller K. B., Schwartz R. S. Familial abnormalities of suppressor-cell function in systemic lupus erythematosus. N Engl J Med. 1979 Oct 11;301(15):803–809. doi: 10.1056/NEJM197910113011502. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Oldstone M. B. Lymphocytes from human newborns abrogate mitosis of their mother's lymphocytes. Nature. 1974 May 10;249(453):161–162. doi: 10.1038/249161a0. [DOI] [PubMed] [Google Scholar]
- Paty D. W., Furesz J., Boucher D. W., Rand C. G., Stiller C. R. Measles antibodies as related to HL-A types in multiple sclerosis. Neurology. 1976 Jul;26(7):651–655. doi: 10.1212/wnl.26.7.651. [DOI] [PubMed] [Google Scholar]
- Rhodes K., Scott A., Markham R. L., Monk-Jones M. E. Immunological sex differences. A study of patients with rheumatoid arthritis, their relatives, and controls. Ann Rheum Dis. 1969 Mar;28(2):104–120. doi: 10.1136/ard.28.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinian J. R., Papoian R., Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977 Jun;59(6):1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Zmijewski C. M., Koprowski H. HLA-related control of spontaneous and antibody-dependent cell-mediated cytotoxic activity in humans. J Immunol. 1976 Sep;117(3):765–770. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R., Fishman J., Brusman H., Kunkel H. G. Systemic lupus erythematosus associated with klinefelter's syndrome. Arthritis Rheum. 1977 Jan-Feb;20(1):18–22. doi: 10.1002/art.1780200103. [DOI] [PubMed] [Google Scholar]
- Stimson W. H., Hunter I. C. Oestrogen-induced immunoregulation mediated through the thymus. J Clin Lab Immunol. 1980 Jul;4(1):27–33. [PubMed] [Google Scholar]
- Talal N. Disordered immunologic regulation and autoimmunity. Transplant Rev. 1976;31:240–263. doi: 10.1111/j.1600-065x.1976.tb01456.x. [DOI] [PubMed] [Google Scholar]
- WHEATER D. W., HURST E. W. The effect of sex on bacterial infections in mice and on the chemotherapy of one of them. J Pathol Bacteriol. 1961 Jul;82:117–130. doi: 10.1002/path.1700820115. [DOI] [PubMed] [Google Scholar]
- Wyle F. A., Kent J. R. Immunosuppression by sex steroid hormones. The effect upon PHA- and PPD-stimulated lymphocytes. Clin Exp Immunol. 1977 Mar;27(3):407–415. [PMC free article] [PubMed] [Google Scholar]


