Abstract
To investigate the possible long-term consequences of gestational exposure to cannabinoids on cognitive functions, pregnant rats were administered with the CB1 receptor agonist WIN 55,212-2 (WIN), at a dose (0.5 mg/kg) that causes neither malformations nor overt signs of toxicity. Prenatal WIN exposure induced a disruption of memory retention in 40- and 80-day-old offspring subjected to a passive avoidance task. A hyperactive behavior at the ages of 12 and 40 days was also found. The memory impairment caused by the gestational exposure to WIN was correlated with alterations of hippocampal long-term potentiation (LTP) and glutamate release. LTP induced in CA3–CA1 synapses decayed faster in brain slices of rats born from WIN-treated dams, whereas posttetanic and short-term potentiation were similar to the control group. In line with LTP shortening, in vivo microdialysis showed a significant decrease in basal and K+-evoked extracellular glutamate levels in the hippocampus of juvenile and adult rats born from WIN-treated dams. A similar reduction in glutamate outflow was also observed in primary cell cultures of hippocampus obtained from pups born from mothers exposed to WIN. The decrease in hippocampal glutamate outflow appears to be the cause of LTP disruption, which in turn might underlie, at least in part, the long-lasting impairment of cognitive functions caused by the gestational exposure to this cannabinoid agonist. These findings could provide an explanation of cognitive alterations observed in children born from women who use marijuana during pregnancy.
Even though marijuana is the most widely used illegal drug among women at reproductive age, reports dealing with the effects of prenatal exposure to this substance of abuse on the length of gestation, fetal growth, and offspring behavior are still controversial (1–4). Confounding factors, such as possible impurities in the drug and concomitant tobacco smoking, may be responsible for inconsistencies in the results reported in studies to date (4, 5). It is likely that many of these conflicting results are due to methodological problems such as the measurement of neonatal outcomes and the context in which the research is conducted. More complex and less understood is the scenario concerning the possible long-term consequences of in utero exposure to cannabis derivatives on cognitive functions. In fact, data on this issue are sparse, and the identification of alterations in brain development and adult expression of cognitive and behavioral functions is far from definitive. These inconclusive results may depend on ethical, practical, and interpretative difficulties surrounding research with human subjects (4). In this regard, animal models provide a useful tool for examining the possible developmental and long-term effects of prenatal exposure to cannabinoids (CBs).
Studies performed in adult rats have demonstrated the involvement of a specific CB receptor (CB1) highly expressed in many brain regions (6) in the reinforcing effects of CBs (7) and also in the disruptive effects of either Δ9-tetrahydrocannabinol (Δ9-THC) or the synthetic agonist WIN 55,212–2 (WIN) (8) on cognitive processes (9).
In particular, it has been reported that deficits of cognitive functions induced by marijuana use during adulthood could be mainly attributable to the activation of CB1 receptors located in the hippocampus (6, 10, 11), a brain region crucial for certain forms of learning and memory. In this regard, it has been shown that CBs decrease excitatory postsynaptic currents and disrupt hippocampal long-term potentiation (LTP) (12–14), which is considered the cellular and molecular model for learning and memory (15, 16).
Accordingly, the CB1 receptor-mediated LTP disruption seems to be associated with an inhibition of hippocampal glutamatergic transmission (14, 17), a finding that could be relevant in elucidating the possible electrophysiological and neurochemical mechanisms underlying the effects of CBs on cognitive functions (11, 18).
The aim of the present study was to determine the effect of long-term prenatal exposure to WIN on cognitive function, hippocampal LTP, and hippocampal glutamate release in juvenile and adult rats. Cognitive function, evaluated with a passive avoidance task, was tested 40 and 80 days after birth. LTP was studied in hippocampal slices obtained from 40-day-old rats. Glutamate release was measured by microdialysis in 40- and 80-day-old rats as well as in hippocampal primary cell cultures obtained from pups born from dams exposed to WIN.
Furthermore, because previous clinical findings have reported abnormal motor activity in children of mothers who used marijuana during pregnancy (3, 19), the effect of prenatal WIN exposure on spontaneous motility was analyzed in infant (12-day-old), juvenile (40-day-old), and adult (80-day-old) offspring.
Materials and Methods
Animal Care.
Experiments were performed in accordance with the guidelines issued by the Italian Ministry of Health (Decreto Legislativo 116/92) and (Decreto Legislativo 111/94-B), the Declaration of Helsinki, and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Animals and Exposure Conditions.
Primiparous Wistar female rats (Harlan SRC, Milan) weighing 250–280 g were housed for 1 week before exposure to males at constant room temperature (20 ± 1°C) and humidity (60%) with lights on 12 h/day (0800 to 2000 h) and food and water available ad libitum. Pairs of females were then placed with single male rats in the late afternoon. Vaginal smears were taken the following morning at 0900 h. The day on which sperm were present was designated as the gestation day 0 (GD 0).
Pregnant rats were treated daily with WIN (0.5 mg/kg) from GD 5 to GD 20. This dose was chosen on the basis of our pilot studies, which showed that prolonged prenatal exposure to a higher WIN dose (1.0 mg/kg) significantly affected reproduction parameters such as dam and pup weight gain as well as litter size at birth. The drug was suspended in 0.3% Tween 80/saline and injected s.c. at the volume of 1.0 ml/kg. Control rats were injected with the vehicle.
Litters were reduced to a standard size of six male pups (when possible) within 24 h after birth. Litters from both control and WIN-exposed groups were then assigned to nonexposed mothers whose pups were born on the same day.
One pup per litter from different litters per treatment group was used in each experiment. Pups were weaned at 21 days of age. Each male pup was used only for a single test and tested once.
Reproduction Data.
Body weights of the dams were taken on GD 0 and GD 20. The number of dams giving birth and the length of pregnancy were determined. Litter size at birth and postnatal mortality (the number of male pups that died before weaning) were evaluated. Body weights of male rats (one pup per litter from 12 control litters and 10 WIN-exposed litters) were recorded.
Behavioral Studies.
Motor activity.
Motor activity was recorded in an Opto-Varimex apparatus linked to an IBM PC (Columbus Instruments, Columbus, OH) according to the method described by Wedzony et al. (20). The apparatus consisted of a cage (42 × 42 × 30 cm) equipped with 15 infrared emitters (spaced at 2.65-cm intervals) located on the x and y axes 2–3 cm above the floor of the cage (depending on the size of the animal) and an equivalent number of receivers located on the opposite walls. A further line of emitter/receiver pairs was located ≈5 cm (depending on the size of the animal) above the floor of the cage to detect vertical movements (i.e., rearings). Each interruption of a beam generates an electric impulse scored by a digital counter.
Procedure.
The amount of time spent in ambulatory activity was analyzed by using AUTO-TRACK software (Columbus Instruments, Columbus, OH). Ambulatory activity was defined as a trespass of three consecutive photo-beams, whereas other movements (e.g., repeated interruption of the same photo-beams) were regarded as stereotypic movements. Resting time was calculated as the amount of time during which there were neither ambulatory nor stereotypic movements. Furthermore, vertical activity was measured by recording the number of horizontal beams that were broken by the rearings of the animal.
Tests (5-min sessions) were carried out in a 1 × 1 × 2 m sound-attenuating cabin (Amplifon G-type cabin) illuminated by a 20-W white light source suspended 2 m above the apparatus. Background noise of 42 dB sound pressure level was produced by a fan. Different groups of animals were tested at 12, 40, and 80 days of age. Experimental groups: (i) vehicle-treated groups (10 animals) and (ii) WIN-treated groups (8 animals). Tests were carried out between 0900 and 1400 h.
Passive avoidance behavior.
A “step-down” apparatus was used according to the method extensively described by Trabace et al. (21). It consisted of a compartment (25 × 24 × 24 cm) constructed of black Plexiglas and equipped with a grid floor to which an elevated compartment (13 × 24 × 16 cm) with a solid Plexiglas floor was attached. A guillotine door (9 × 10 cm) separated the opening between the elevated compartment and the large compartment. A 25-W lamp illuminated the elevated compartment while the large compartment remained dark. Scrambled foot shocks were delivered from a Letica shock generator (LI 2750 Unit, Barcelona). The experiments were performed in a sound-attenuating chamber (Amplifon G-type cabin) that was dark except for illumination of the elevated compartment of the apparatus.
Procedure.
Each animal was removed from the home cage and placed in a holding cage adjacent to the apparatus. Two minutes later, the rat was placed in the illuminated compartment, and, after a 10-s delay, the guillotine door was raised. The time taken by the animal to completely enter into the dark compartment was measured (approach latency) and taken as an index of emotional, nonassociative behavior.
A single 2-s inescapable scrambled foot shock (0.8 mA) was delivered immediately after the rat entered the dark compartment. Twenty-four hours after this session (acquisition trial), each animal was tested for memory retention. The animal was placed in the elevated compartment and latency to re-enter (avoidance latency) the dark compartment was recorded and assumed to be a measure of memory retention. Both acquisition and retention trials lasted for a maximum observation time of 180 s. The experiments were conducted in 40- and 80-day-old male offspring of either control or WIN-exposed mothers, each group consisting of eight rats.
Electrophysiological Studies.
The electrophysiological experiments (see ref. 22 for details) were performed in 40-day-old offspring of either vehicle-exposed (n = 10) or WIN-exposed (n = 9) mothers. Transverse hippocampal slices were prepared following standard methods. Briefly, rats were decapitated under deep anesthesia by halothane (4.0% in O2), and the brain was rapidly removed. Slices (350 μm thick) were cut in chilled Ringer solution with a vibroslicer (VSL, WPI, Sarasota, FL), incubated at room temperature (20 ± 2°C) for at least 60 min, and then individually transferred to the recording (submerged) chamber. At least two slices from each animal were tested. Ringer medium contained 124 mM NaCl, 3.5 mM KCl, 1.25 mM NaH2PO4, 22.0 mM NaHCO3, 10.0 mM dextrose, 1.0 mM MgCl2, 2.0 mM CaCl2. The solution was maintained at pH 7.4 by continuous bubbling with 5% CO2 in O2.
The procedure was as follows. Field-excitatory postsynaptic potentials (f-EPSPs) were recorded from stratum radiatum of CA1 pyramidal cells in response to monopolar stimuli (20 μs-duration) delivered to the Schaffer collateral/commissural pathway via platinum electrodes. Recording electrodes were filled with the medium (1–2 MΩ). Synaptic responses were sampled at 5–10 kHz. Acquisition and analysis were performed by a pCLAMP 5.5/Digidata 1200 system (Axon Instruments Inc., Foster City, CA). The evoked f-EPSPs were measured as the slope of their rising phase after the presynaptic volley. An I/O curve was constructed for each slice by plotting increasing single stimulus intensity (scan: 50 to 1,000 μA) vs. the evoked f-EPSP. The current intensity required to produce 50% of maximal response (EC50) was used to assess the synaptic excitability and was used for test stimulation and tetanization.
Samples of f-EPSP were taken every 5 min, averaging 10 consecutive responses (22). Tetanization consisted of two trains of stimuli (100 Hz for 1.0 s at 25-s intervals) delivered after at least 30 min of baseline. Responses were followed up to 180 min and were considered potentiated if their slope was ≥20% of baseline.
The three temporal phases of f-EPSP changes, i.e., posttetanic potentiation (PTP), short-term potentiation (STP), and LTP expression (or maintenance) were distinguished as indicated (15, 16, 22, 23).
Neurochemical Studies.
Microdialysis.
In vivo experiments were performed in the offspring of WIN-treated and vehicle-treated dams, at the age of 40 and 80 days. Under halothane anesthesia (1.5% mixture of halothane/air), animals were mounted in a David Kopf stereotaxic apparatus, and a microdialysis probe (1 mm dialyzing membrane length) was implanted into the hippocampus. The coordinates relative to bregma were as follows: anteroposterior, −5.2; mediolateral, ±4.0; and dorsoventral, −3.8 mm (24). After the implantation, the probe was secured to the skull with methacrylic cement. Microdialysis measures were performed after at least 36 h of recovery.
Procedure.
On the day of the experiment, the probe was perfused with an artificial cerebrospinal fluid (148 mM NaCl/2.7 mM KCl/1.2 mM CaCl2/0.85 mM MgCl2/2.7 mM glucose) at a constant flow rate (2 μl/min) via a microinfusion pump. At least 300 min later, dialysates were collected every 20 min, and glutamate content was measured by HPLC. The average concentration of three successive stable samples (variation ≤10%) was considered as baseline glutamate outflow. Thereafter, the probe was perfused (10 min) with an isotonic artificial cerebrospinal fluid containing 50 mM KCl. This medium was then replaced with the original one, and further four samples were collected.
Histology.
At the end of each experiment, the probe location was verified in 30-μm-thick coronal cryostat sections. Only those animals in which the probe was correctly located were included in the study.
Hippocampal cell cultures.
Hippocampal cells were prepared from 1-day-old rats (25) born from mothers that had received the WIN-vehicle (control) or WIN during pregnancy. Briefly, neurons were plated on poly-l-lysine (5 μg/ml)-coated dishes at a density of 2.5 × 106 cells per dish and cultured in Eagle's Basal Medium supplemented with inactivated FCS, 25 mM KCl, 2 mM glutamine, and 100 μg/ml gentamycine. Cultures were grown at 37°C in a humidified atmosphere, 5% CO2/95% air. Cytosine arabinoside (10 μM) was added within 24 h of plating to prevent glial cell replication. The cultures were used in experiments after 8 days in vitro.
Procedure.
On the day of the experiment, the cells were rinsed twice by replacing the culture medium with Krebs–Ringer bicarbonate buffer (37°C). Thereafter, five consecutive fractions were collected renewing this solution (400 μl) every 30 min. The first three samples were used to assess basal glutamate levels while, to evoke endogenous glutamate, cells were treated with an isotonic Krebs solution containing 20 mM KCl, applied 20 min before the end of the fourth fraction.
Endogenous glutamate assay.
Endogenous glutamate was quantified by using an HPLC/fluorimetric detection system, including precolumn derivatization o-phtaldialdeihyde reagent and a Chromsep 5 (C18) column. The mobile phase consisted of 0.1 M sodium acetate, 10% methanol, and 2.5% tetrahydrofurane, pH 6.5 (0.75 ml/min; ref. 26).
Statistical Analysis.
The reproduction data were analyzed by overall one-way or two-way ANOVAs followed by post hoc tests (Tukey's test) for individual comparisons between groups. Fisher's exact test was used where appropriate.
The analysis of motor activity data were based on overall two-way ANOVAs followed by post hoc tests (Tukey's test).
Mann–Whitney U test was used to analyze the passive avoidance data. The electrophysiological results were evaluated by a two-way ANOVA for repeated measures followed by Tukey's test or Student's t test, where appropriate. Data obtained from neurochemical studies were analyzed by Student's t test for grouped data.
Substances.
WIN mesylate {(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl)pyrrolo (1,2,3-de)-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone} was obtained from Tocris Cookson (Bristol, U.K.). The culture dishes were purchased from Nunc. FCS and basal Eagle's medium were obtained from GIBCO. Poly-l-lysine, trypsin, soybean trypsin inhibitor, DNase, cytosine arabinoside, gentamycine sulfate, and glutamine were obtained from Sigma.
Results
General Reproduction Data.
General reproduction data are reported in Table 1. Overall one-way ANOVAs showed that prenatal treatment with WIN did not significantly affect dam weight gain [F = 3.65, df = 1/20, not significant (n.s.)], pregnancy length (F = 0.33, df = 1/20, n.s.), and litter size at birth (F = 1.71, df = 1/20, n.s.). Moreover, an overall two-way ANOVA for repeated measures showed that prenatal exposure to the CB1 receptor agonist did not influence male pup weight gain: (Ftreatments = 0.01, df = 1/20, n.s.; Fages = 1638, df = 2/40, P < 0.001; Ftreatments×ages = 0.52, df = 2/40, n.s.). Finally, Fisher's exact test revealed that WIN treatment caused neither hypothermia, catatonia, or hypomotility in dams, nor postnatal toxicity or teratogenesis in male pups (data not shown).
Table 1.
Reproduction data
| Group | Dam weight gain*, % | Pregnancy length, days | Litter size at birth | Pup weight gain
|
||
|---|---|---|---|---|---|---|
| PND 1 | PND 14 | PND 21 | ||||
| Vehicle | 54.4 ± 1.0 | 21.0 ± 0.13 | 11.25 ± 0.55 | 6.48 ± 0.15 | 32.55 ± 1.05 | 75.10 ± 0.41 |
| WIN | 50.6 ± 3.0 | 20.8 ± 0.13 | 12.30 ± 0.60 | 6.34 ± 0.16 | 31.66 ± 0.65 | 74.11 ± 0.32 |
Data represent mean values ± SEM.
From day 0 to day 20 of pregnancy.
Behavioral Studies.
Motor activity.
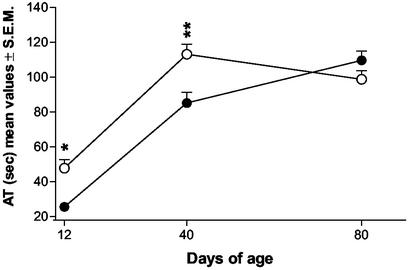
An overall two-way ANOVA for repeated measures of the ambulatory time (Fig. 1) showed the following effects: Ftreatments = 12.72, df = 1/16, P < 0.005; Fages = 100.18, df = 2/32, P < 0.001; Ftreatments×ages = 7.80, df = 2/32, P < 0.001. Individual comparisons (Tukey's test) revealed that prenatal treatment with WIN significantly increased the ambulatory time of the offspring at both postnatal day (PND) 12 (P < 0.05) and 40 (P < 0.01). No significant differences were observed in ambulatory activity between the two groups at 80 days of age.
Figure 1.
Effects of prenatal treatment with WIN on motor activity in 12-, 40-, and 80-day-old rats (●, vehicle; ○, WIN). Each point represents the mean ± SEM of the ambulatory time (AT) spent by rats in 5-min trials. n was 10 and 8 for vehicle- and WIN-exposed rats, respectively. *, P < 0.05; **, P < 0.01 (vs. controls; Tukey's multiple comparison test).
Furthermore, overall two-way ANOVAs for repeated measures of both stereotypic time and rearings did not reveal any significant change between controls and WIN-exposed offspring at all ages (PND 12, 40, and 80) considered in the present study (data not shown).
Passive avoidance behavior.
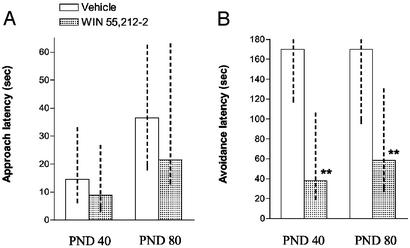
As shown in Fig. 2, during the first (acquisition) trial 40- and 80-day-old rats from the control group showed approach latencies that did not differ significantly with respect to animals prenatally exposed to WIN. However, when the trial was repeated 24 h later (retention trial), the avoidance latencies of the WIN-exposed group were significantly shorter than those of control animals (P < 0.01, Mann–Whitney U test).
Figure 2.
Effect of prenatal WIN exposure on approach latency (A) and avoidance latency (B) measured 24 h later (retention) of 40- and 80-day-old offspring in a passive avoidance task. Data represent median values and interquartiles (dashed line). **, P < 0.01 with respect to relative control (Mann–Whitney U test).
Electrophysiological Studies.
Synaptic excitability.
Changes in basal synaptic excitability in hippocampal slices from 40-day-old rats were investigated, comparing the current intensity required to produce 50% of maximal response (excitatory current 50) before tetanization in both groups and by the evaluation of the number of slices exhibiting PTP.
Although the EC50 in slices from WIN-treated rats (WIN-slices) was found to be slightly higher than it was in controls, no statistical significance was reached (Student's t test). This result indicates that the responsiveness of CA3–CA1 synapses to electrical stimuli was not affected by the treatment.
Moreover, the first potentiation, which immediately follows tetanization (PTP), was found to be comparable in the two groups [255.90 ± 18.07 and 229.18 ± 23.43% for vehicle- and WIN-treated animals, respectively (Student's t test)]. Furthermore, the occurrence of slices showing a PTP of at least 200% was similar [22/22 and 21/21 in slices from control and WIN-treated group, respectively (Table 2)].
Table 2.
Number of slices from 30- to 40-day-old offspring showing PTP, STP, and LTP of f-EPSP
| Vehicle-exposed (n* = 22, from 10 rats)
|
WIN-exposed (n* = 21, from 9 rats)
|
|||
|---|---|---|---|---|
| n† | % | n† | % | |
| PTP | 22 | 100.00 | 21 | 100.00 |
| STP | 22 | 100.00 | 17 | 80.95 |
| LTP | 20 | 90.91 | 3 | 14.29‡ |
Values indicate the occurrence of tests where slices exhibited temporal changes of EPSP as described in Materials and Methods.
Number of slices tested.
†Number of slices exhibiting changes.
‡ P < 0.001 with respect to vehicle (Fisher's exact test).
Taken together, these results indicate that no alterations in basal synaptic excitability were evident in slices from prenatally WIN-exposed rats.
Time course of STP and LTP.
In control slices the decay of f-EPSP potentiation after tetanization followed a typical biphasic curve (Fig. 3A). Thus, in agreement with previous studies (15, 16, 23, 27), the f-EPSP slope in control slices showed a first fast decremental phase lasting 15 to 20 min (STP), which then slowly decayed over the observation time (180 min).
Figure 3.
Prenatal WIN selectively suppresses LTP maintenance but not its induction. (A) Time course of the averages of f-EPSP slopes from slices obtained from 40-day-old offspring of vehicle-treated (●) and WIN-treated dams (○). Values of f-EPSP slopes have been normalized to the pretetanus period. Each point represents the average ± SEM of 10 consecutive responses taken every 5 min. Just after tetanization (given at time −5 min), the values of f-EPSP first potentiation (i.e., the PTP) were 255.90 ± 18.07 and 229.18 ± 23.43% for vehicle- and WIN-treated groups, respectively (not significant, Student's t test). (B) Duration of LTP. Evoked f-EPSPs were considered potentiated until their slope was ≥20% with respect to baseline. For the vehicle group, LTP duration was estimated by fitting analysis of the curve in A to calculate the interception point where this curve asymptotically subsided to a value of +20%. It occurred at 334.45 ± 25.36 min after tetanus. However, the curve describing LTP expression of the WIN-treated group (data from A) returned to +20% of baseline in 136.87 ± 12.18 min. Bars represent the mean ± SEM obtained from 22 and 21 slices from vehicle- and WIN-exposed rats, respectively. *, P < 0.001 vs. vehicle (Student's t test).
In line with the PTP results (see above), the time course of the early phase of f-EPSP de-potentiation (STP) was similar in both treated and control groups (Fig. 3A). Indeed, a two-way ANOVA showed no significant deviation between the two curves (P < 0.09) in the STP interval (from 5 to 20 min).
Thereafter, however, the averaged curve describing the LTP-expression phase decayed faster in slices from WIN-treated than in vehicle-treated offspring (Fig. 3A). Accordingly, the deviation between the two curves, in the 20- to 180-min interval after tetanization, was statistically significant (P < 0.01, two-way ANOVA).
Moreover, whereas a typical time course of LTP was seen in 20/22 tested slices from control animals, the potentiation remained above +20% for >60 min in only 3 of 21 slices from the WIN group (Table 2).
As shown by fitting analysis, the interception points at which the averaged curves of LTP, asymptotically subsided to a value of +20%, with respect to the baseline, were at 334.45 ± 25.36 and 136.87 ± 12.18 min, after tetanus, in control and WIN slices, respectively (Fig. 3B).
Neurochemical Studies.
Hippocampal cell cultures experiments.
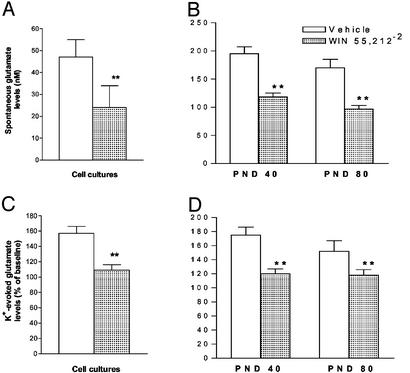
Basal extracellular glutamate levels were measured in hippocampal cell cultures obtained from 1-day-old pups. As shown in Fig. 4A, glutamate levels were found to be significantly lower (P < 0.01; Student's t test) in animals born from mothers exposed to WIN during pregnancy than in those born from vehicle-treated mothers.
Figure 4.
Effect of prenatal exposure to WIN on basal (A and B) and K+-evoked (C and D) extracellular hippocampal glutamate levels in cell cultures (A and C) and in vivo at 40 and 80 days of age (B and D). Each column represents the mean ± SEM obtained from 22 and 11 replications for in vitro and in vivo experiments, respectively. **, P < 0.01 vs. vehicle (Student's t test).
Bath application of KCl (20 mM) increased glutamate extracellular levels in both cell cultures. However, the increase was significantly lower (P < 0.01; Student's t test) in cultures of rats born from WIN-treated mothers than in those obtained from control pups (Fig. 4C).
Microdialysis in vivo.
Basal extracellular hippocampal glutamate levels, evaluated as the mean of three stable dialysates, were significantly lower (P < 0.01; Student's t test) in both 40- and 80-day-old rats born from mothers treated with WIN during pregnancy than in those born from mothers treated with the vehicle (Fig. 4B).
A 10-min pulse of high K+ (50 mM) solution significantly increased glutamate efflux in both groups of animals. However, the K+-evoked glutamate efflux from the hippocampus of rats born from mothers exposed to WIN during pregnancy, was significantly lower (P < 0.01; Student's t test) than the enhancement observed in rats born from mothers treated with vehicle during pregnancy (Fig. 4D).
Discussion
The present study, by combining different methodological approaches, provides evidence that maternal exposure to the CB1 receptor agonist WIN induces impairment of memory retention capacities in the offspring. This impairment is associated with alterations of hippocampal LTP and glutamate outflow.
WIN-exposed offspring were also characterized by motor hyperactivity during infantile and juvenile, but not adult, periods.
Memory impairment in prenatally WIN-exposed rats, assessed by the disruption in the retention of a passive avoidance task, seems to be a persistent condition, present at both 40 and 80 days of age.
Memory impairment does not appear to be attributable to alterations of a nonassociative nature, because approach latency, measured during the acquisition trials of the learning task, remained unchanged.
WIN-treated dams did not show hypothermia, catatonia, or hypomotility, which are typically induced by the high and/or moderate exposure to CBs (8). Moreover, the dose of WIN used in the present study (0.5 mg/kg/die s.c.) produced neither gross malformations nor overt signs of toxicity, and it failed to alter reproductive parameters, such as dam and pup weight and weight gain. Furthermore, litters of WIN-treated dams were assigned to untreated dams to avoid confounding factors generated during lactation as well as malnutrition.
However, memory deficit produced by prenatal WIN may be dissociated from the hyperactivity, which has been reported to be caused postnatally by WIN (28), because the latter was present at 40 but not at 80 days of age, whereas the former was present at both periods.
Memory impairment observed in offspring exposed prenatally to WIN was correlated to alterations in both hippocampal LTP, a widely accepted cellular and molecular model for learning and memory (15, 16), and hippocampal glutamate release.
LTP was assessed in brain slices from 40-day-old rats, whereas spontaneous and K+-evoked glutamate release was measured in vivo at 40 and 80 days of age, as well as in cell cultures obtained from 1-day-old pups.
Slices from WIN-treated animals showed a reduced ability to maintain LTP over time, whereas basal synaptic excitability and LTP induction phases (PTP and STP) were normal.
These results are in agreement with previous observations made in brain slices from adult rats showing that bath application of low concentrations of Δ9-THC selectively reduced LTP duration but not the extent of PTP (12).
However, other authors have reported that CB1 receptor activation by WIN, in slices from adult rodent brain, suppressed both early (induction) and late (maintenance/expression) phases of LTP in hippocampal CA3-CA1 synapses (11, 14, 29). The inhibitory effect of CBs on hippocampal LTP has been attributed to the reduction in presynaptic glutamate release and the consequent suppression of N-methyl-d-aspartate-mediated entry of postsynaptic Ca2+, necessary for LTP induction, rather than to a direct modulation of postsynaptic ionic channels (11, 14, 30).
According to this hypothesis, the microdialysis data have shown that basal and K+-stimulated extracellular hippocampal glutamate release was significantly lower in animals born from WIN-treated dams than in control animals.
Therefore, it might be suggested that in rats exposed prenatally to WIN, glutamate release is sufficiently preserved to activate N-methyl-d-aspartate receptors responsible for LTP induction, but it is not sufficiently sustained to stimulate postsynaptic metabotropic and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors involved in LTP maintenance (15).
The reduced glutamate outflow seems to be a precocious and persisting consequence of prenatal exposure to the CB agonist; it is already present in cell cultures, obtained from 1-day-old WIN-exposed pups, and persists unmitigated at 40 and 80 days of age.
Although the reduction of glutamate outflow could explain the disruption of LTP induced by gestational exposure to the CB1 receptor agonist, the electrophysiological alterations may, in turn, represent a neuronal substrate responsible for the selective retention deficit (reduction of avoidance latencies in a passive avoidance task) that was observed in the offspring of mothers treated with WIN during pregnancy.
Thus, it may be hypothesized that gestational exposure to the CB produces an irreversible alteration to endogenous CB1 systems in the developing brain (29, 31), possibly leading to a long-term disruption of hippocampal function. Accordingly, CB1 receptors are already measurable at GD 14 in a variety of brain structures including hippocampus (32).
Additional studies are needed to clarify whether the effects caused by WIN are reproduced by Δ9-THC and whether they may be prevented by CB1 receptor antagonists such as SR 141716.
Concerning the clinical relevance of the present study, it is important to estimate, by extrapolation, whether the dose of WIN administered compares with that of Δ9-THC absorbed by cannabis users.
Previous studies have estimated that 5 mg/kg Δ9-THC in rats corresponds to a moderate exposure of the drug in humans, correcting for the differences in route of administration and body weight surface area (33, 34, 35).
However, WIN has been found to be 3–10 times more potent than Δ9-THC, depending on the administration route and the endpoints considered (8, 36, 37). This estimate is consistent with the relative Ki of each compound for CB1 receptors in brain membranes, i.e., 2–12 nM vs. 35–80 nM for WIN and Δ9-THC, respectively (38). Based on these considerations, the dose of WIN used in the present study might correspond to a moderate, or even to a low, exposure to cannabis in humans.
The present results are in line with clinical data showing that the consumption of marijuana by women during pregnancy has negative consequences on the cognitive functions of their children. In particular, memory has been reported to be negatively associated with daily marijuana use, and this statistical association remained after checking for confounding variables (39).
Moreover, the increased motor activity observed in both infant and juvenile offspring of WIN-treated dams is consistent with data showing that children prenatally exposed to marijuana were rated, at a prepuberty age, as hyperactive, inattentive, and particularly impulsive (3, 19).
Whatever the mechanism of action of prenatal exposure to WIN, our results suggest that alterations of hippocampal glutamategic function may underlie, at least in part, the subtle impairment of cognitive processes induced by gestational marijuana exposure (1, 4, 39).
Acknowledgments
We thank Dr. Alessandra Meloni and Dr. Paola Salis for their assistance in the electrophysiological experiments. This study was supported by Ministero dell'Istruzione, dell'Università, e della Ricerca (Progetti di Ricerca di Rilevanza Nazionale and Fondo per gli Investimenti della Ricerca di Base 2002) and Consiglio Nazionale delle Ricerche–Agenzia 2000 (CNRC002514) grants.
Abbreviations
- CB
cannabinoid
- f-EPSP
field excitatory postsynaptic potential
- LTP
long-term potentiation
- PTP
posttetanic potentiation
- STP
short-term potentiation
- GD
gestation day
- PND
postnatal day
- WIN
WIN 55,212-2
- Δ9-THC
Δ9-tetrahydrocannabinol
References
- 1.Day N L, Richardson G A, Goldschmidt L, Robles N, Taylor P M, Stoffer D S, Cornelius M D, Geva D. Neurotoxicol Teratol. 1994;16:169–175. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 2.Navarro M, Rubio P, Rodríguez de Fonseca F. Psychopharmacology (Berl) 1995;122:1–14. doi: 10.1007/BF02246436. [DOI] [PubMed] [Google Scholar]
- 3.Goldschmidt L, Day N L, Richardson G A. Neurotoxicol Teratol. 2000;22:325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 4.Fried P A, Smith A M. Neurotoxicol Teratol. 2001;23:1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 5.Cornelius M D, Taylor P M, Geva D, Day N L. Pediatrics. 1995;95:738–743. [PubMed] [Google Scholar]
- 6.Howlett A C, Bidaut-Russel M, Devane W A, Melvin L S, Johnson M R, Herkenham M. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 7.Fattore L, Cossu G, Martellotta C M, Fratta W. Psychopharmacology (Berl) 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- 8.Compton D R, Gold L H, Ward S J, Balster R L, Martin B R. J Pharmacol Exp Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- 9.Varvel S A, Lichtman A H. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- 10.Hall W, Solowij N. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan J M. Learn Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- 12.Nowicky A V, Teyler T J, Vardaris R M. Brain Res Bull. 1987;19:663–672. doi: 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 13.Collins D R, Pertwee R G, Davies S N. Eur J Pharmacol. 1994;259:R7–R8. doi: 10.1016/0014-2999(94)90666-1. [DOI] [PubMed] [Google Scholar]
- 14.Misner D L, Sullivan J M. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliss T V P, Collingridge G L. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 16.Nicoll R A, Malenka R C. Nature. 1995;377:31–39. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 17.Piomelli D, Giuffrida A, Calignano A, Rodríguez de Fonseca F. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 18.Shen M, Piser T, Seybold V S, Thayer S A. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried P A, Watkinson B, Gray R. Neurotoxicol Teratol. 1992;14:299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- 20.Wedzony K, Mackowiak M, Zajaczkowski W, Fijal K, Chocyk A, Czyrak A. Neuropsychopharmacology. 2000;23:547–559. doi: 10.1016/S0893-133X(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 21.Trabace L, Cassano T, Steardo L, Pietra C, Villetti G, Kendrick K M, Cuomo V. J Pharmacol Exp Ther. 2000;294:187–194. [PubMed] [Google Scholar]
- 22.Mereu G, Cammalleri M, F, Francesconi W, Saba P, Tattoli M, Trabace L, Vaccari A, Cuomo V. J Pharmacol Exp Ther. 2000;294:728–734. [PubMed] [Google Scholar]
- 23.Schulz P E, Fitzgibbons J C. J Neurophysiol. 1997;78:321–334. doi: 10.1152/jn.1997.78.1.321. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Ed. New York: Academic; 1986. , figure 26. [DOI] [PubMed] [Google Scholar]
- 25.Alho H, Ferrarese C, Vicini S, Vaccarino F. Dev Brain Res. 1988;39:193–204. doi: 10.1016/0165-3806(88)90023-5. [DOI] [PubMed] [Google Scholar]
- 26.Ferraro L, Tomasini M C, Gessa G L, Bebe B W, Tanganelli S, Antonelli T. Cereb Cortex. 2001;11:728–733. doi: 10.1093/cercor/11.8.728. [DOI] [PubMed] [Google Scholar]
- 27.Manabe T, Wyllie D J A, Perkel D J, Nicoll R A. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 28.Wolf C, Almli C R, Finger S, Ryan S, Morgane P J. Physiol Behav. 1986;38:725–730. doi: 10.1016/0031-9384(86)90270-2. [DOI] [PubMed] [Google Scholar]
- 29.Terranova J P, Michaud J C, Le Fur G, Soubrie P. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- 30.Kim D J, Thayer S A. Brain Res. 2000;852:398–405. doi: 10.1016/s0006-8993(99)02210-6. [DOI] [PubMed] [Google Scholar]
- 31.Fride E, Mechoulam R. Psychoneuroendocrinology. 1996;21:157–172. doi: 10.1016/0306-4530(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 32.Berrendero F, García-Gil L, Hernández M L, Romero J, Cebeira M, de Miguel R, Ramos J A, Fernández-Ruiz J J. Development (Cambridge, UK) 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- 33.Rosenkrantz H, Braude M C. In: The Pharmacology of Marijuana. Braude M C, Szara S, editors. New York: Raven; 1976. p. 571. [Google Scholar]
- 34.García-Gil L, De Miguel R, Munoz R M, Cebeira M, Villanua M A, Ramos J A, Fernández-Ruiz J J. Neurotoxicol Teratol. 1997;19:477–487. doi: 10.1016/s0892-0362(97)00048-2. [DOI] [PubMed] [Google Scholar]
- 35.García-Gil L, Ramos J A, Rubino T, Parolaro D, Fernández-Ruiz J J. Neurotoxicol Teratol. 1998;20:549–553. doi: 10.1016/s0892-0362(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 36.French E D, Dillon K, Wu X. NeuroReport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 37.Hampson R E, Deadwyler S A. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pertwee R. Exp Opin Invest Drugs. 2000;9:1553–1571. doi: 10.1517/13543784.9.7.1553. [DOI] [PubMed] [Google Scholar]
- 39.Fried P A, Watkinson B. J Dev Behav Pediatr. 1990;11:49–58. [PubMed] [Google Scholar]