Abstract
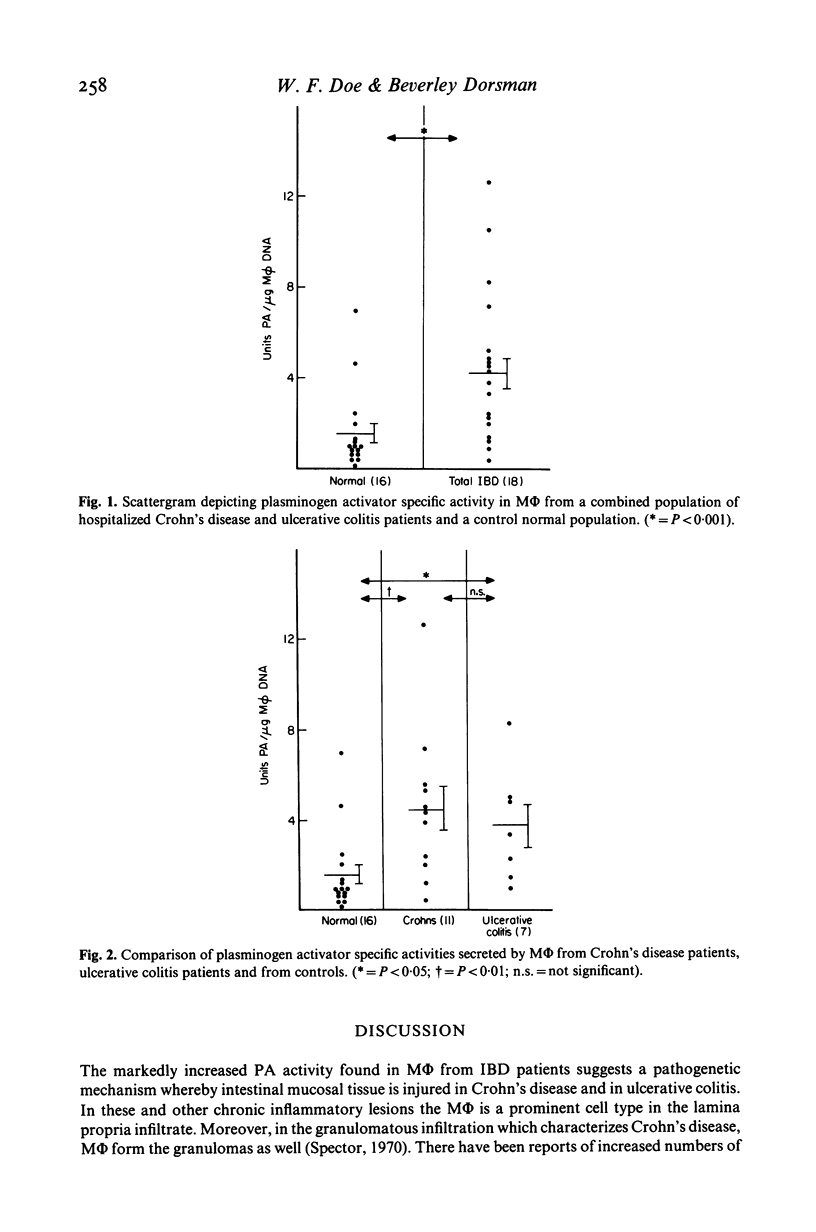
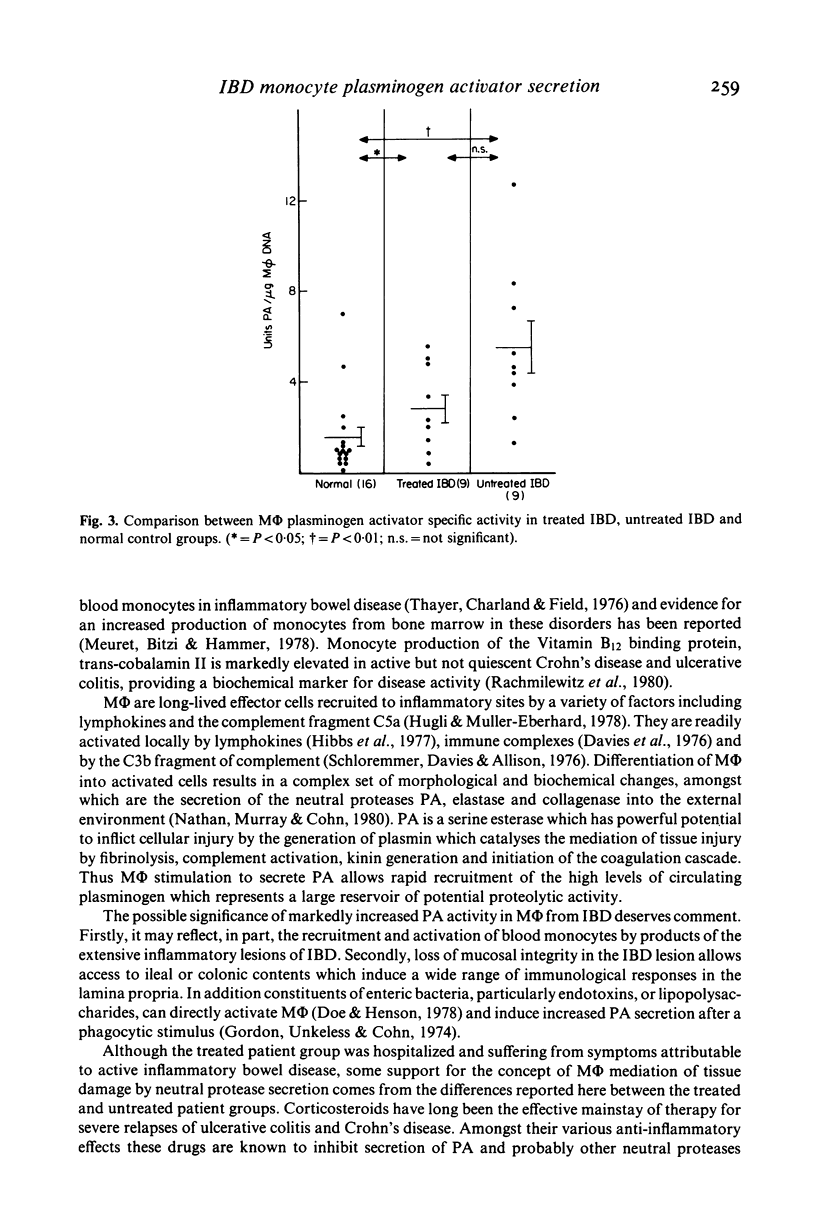
The secretion of the neutral protease plasminogen activator (PA) by cultured macrophages (M phi) was studied in hospitalized patients suffering from chronic inflammatory bowel disease (IBD). There was markedly enhanced secretion of PA by M phi derived from circulating monocytes of the IBD population (18) compared to an age-matched population (16) which was not afflicted by intestinal disease (P less than 0 . 001). Mean M phi PA activity was greater in the population of 11 Crohn's disease patients (P less than 0 . 01) than in a group of seven ulcerative colitis sufferers (P less than 0 . 05) when compared to the control population. While both the treated and untreated hospitalized IBD populations showed increased M phi PA specific activity, results for the nine untreated patients (5 . 56 +/- 1.14 units/micrograms M phi DNA) were substantially higher than those found in the treated IBD population (2 . 91 +/- 0 . 62 units/micrograms M phi DNA) (P less than 0 . 01). These findings reflect the activity of M phi in IBD and suggest a means by which tissue injury is mediated in these conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Unkeless J. C., Cohn Z. A. Induction of macrophage plasminogen activator by endotoxin stimulation and phagocytosis: evidence for a two-stage process. J Exp Med. 1974 Oct 1;140(4):995–1010. doi: 10.1084/jem.140.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Meuret G., Bitzi A., Hammer B. Macrophage turnover in Crohn's disease and ulcerative colitis. Gastroenterology. 1978 Mar;74(3):501–503. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Ligumsky M., Rachmilewitz B., Rachmilewitz M., Tarcic N., Schlesinger M. Transcobalamin II level in peripheral blood monocytes--a biochemical marker in inflammatory diseases of the bowel. Gastroenterology. 1980 Jan;78(1):43–46. [PubMed] [Google Scholar]
- Ratnoff O. D., Naff G. B. The conversion of C'IS to C'1 esterase by plasmin and trypsin. J Exp Med. 1967 Feb 1;125(2):337–358. doi: 10.1084/jem.125.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Davies P., Allison A. C. Ability of activated complement components to induce lysosomal enzyme release from macrophages. Nature. 1976 May 6;261(5555):48–49. doi: 10.1038/261048a0. [DOI] [PubMed] [Google Scholar]
- Spector W. G. The macrophage in inflammation. Ser Haematol. 1970;3(2):132–144. [PubMed] [Google Scholar]
- Thayer W. R., Jr, Charland C., Field C. E. The subpopulations of circulating white blood cells in inflammatory bowel disease. Gastroenterology. 1976 Sep;71(3):379–384. [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. Biochemical actions of glucocorticoids on macrophages in culture. Specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med. 1978 Jun 1;147(6):1695–1712. doi: 10.1084/jem.147.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]



