Abstract
Several chemical changes in soil are associated with plant growth-promoting rhizobacteria (PGPR). Some bacterial strains directly regulate plant physiology by mimicking synthesis of plant hormones, whereas others increase mineral and nitrogen availability in the soil as a way to augment growth. Identification of bacterial chemical messengers that trigger growth promotion has been limited in part by the understanding of how plants respond to external stimuli. With an increasing appreciation of how volatile organic compounds signal plants and serve in plant defense, investigations into the role of volatile components in plant–bacterial systems now can follow. Here, we present chemical and plant-growth data showing that some PGPR release a blend of volatile components that promote growth of Arabidopsis thaliana. In particular, the volatile components 2,3-butanediol and acetoin were released exclusively from two bacterial strains that trigger the greatest level of growth promotion. Furthermore, pharmacological applications of 2,3-butanediol enhanced plant growth whereas bacterial mutants blocked in 2,3-butanediol and acetoin synthesis were devoid in this growth-promotion capacity. The demonstration that PGPR strains release different volatile blends and that plant growth is stimulated by differences in these volatile blends establishes an additional function for volatile organic compounds as signaling molecules mediating plant–microbe interactions.
Plant growth-promoting rhizobacteria (PGPR) are naturally occurring soil microorganisms that colonize roots and stimulate plant growth. Such bacteria have been applied to a wide range of agricultural species for the purposes of growth enhancement, including increased seed emergence, plant weight, crop yields, and disease control (1, 2). For example, emergence increases of 10–40% resulted for canola when seeds were coated with PGPR before planting (1), and plant weight of tuber-treated potatoes increased by 80% on average by midseason (3). Yield increases between 10% and 20% with PGPR applications have been documented for several agricultural crops (1).
Proposed mechanisms for plant-growth promotion by PGPR include bacterial synthesis of the plant hormones indole-3-acetic acid (4), cytokinin (5), and gibberellin (6); breakdown of plant-produced ethylene by bacterial production of 1-aminocyclopropane-1-carboxylate deaminase (7); and increased mineral and N availability in the soil (8). Although low-molecular-weight plant volatiles such as terpenes, jasmonates, and green leaf components have been identified as potential signal molecules for plants and organisms of other trophic levels (9, 10), the role volatile emissions from bacteria play in plant development is unknown.
Here, we report that a blend of airborne chemicals released from specific strains of PGPR promotes growth of Arabidopsis thaliana seedlings. Analysis of the volatiles emitted from two of the most potent growth-promoting strains, Bacillus subtilis GB03 and Bacillus amyloliquefaciens IN937a, revealed that two compounds, 3-hydroxy-2-butanone (acetoin) and 2,3-butanediol, were shared by both bacterial strains whereas other PGPR strains that did not trigger enhanced growth via volatile emissions also did not share this same subset of volatile components. Acetoin synthesis in B. subtilis involves the enzymes acetolactate synthase (pyruvate to acetolactate) and acetolactate decarboxylase (acetolactate to acetoin), which are encoded by alsS and alsD, respectively. The first two genes in this pathway are organized in a previously characterized operon, whereas the gene that converts acetoin to 2,3-butanediol (the acetoin-reductase step) presently is unknown (11). Most interestingly, insertional inactivation of the als operon (strain BSIP1173) has only a small effect on the growth behavior of B. subtilis under a variety of conditions tested (11), whereas the mutation totally abolished 2,3-butanediol production (11) and negated all growth-promotion effects triggered by airborne signals. Confirmation that this four-carbon volatile alcohol indeed does trigger plant growth promotion was provided by the exogenous application of 2,3-butanediol to A. thaliana seedlings that resulted in dose-dependent growth promotion.
Materials and Methods
Bacterial Cultures.
Seven strains of PGPR (all from Auburn University) were used, including Pseudomonas fluorescens 89B-61, Bacillus pumilus T4, B. pasteurii C-9, B. subtilis GB03, B. amyloliquefaciens IN937a, Serratia marcescens 90–166, and Enterobacter cloacae JM22. The nongrowth-promoting strain Escherichia coli DH5α (Qiagen, Chatsworth, CA) was used as a control. Other bacteria used included B. subtilis 168 (Bacillus Genetic Stock Center, Ohio State University, Columbus) and B. subtilis strains BSIP1171 [2,3-butanediol-producing (11)], BSIP1173 [2,3-butanediol nonproducing (11)], and BSIP1174 [2,3-butanediol nonproducing (11)], provided by D. Jahn (Braunschweig University, Braunschweig, Germany). For experimental use, all bacteria were streaked onto tryptic soy agar plates (Difco) that contained 2% agar and incubated at 28°C in the absence of light. For long-term storage, bacterial cultures were maintained at −80°C in tryptic soy agar that contained 20% glycerol.
Plant Material.
A. thaliana seeds were surface-sterilized (2-min, 70% ethanol soaking followed by a 20-min, 1% sodium hypochlorite soaking), rinsed (four times) in sterile, distilled water, placed on petri dishes containing half-strength Murashige and Skoog salt (MS) medium (GIBCO/BRL) containing 0.8% agar and 1.5% sucrose, adjusted to pH 5.7, and vernalized for 2 days at 4°C in the absence of light. Seedlings then were placed in growth cabinets (Sanyo Scientific, Itasca, IL) set to a 12-h-light/12-h-dark cycle under 40-W fluorescent lights; the temperature was maintained at 22 ± 1°C with a relative humidity of 50–60%. Germinated seedlings were transferred after 2 days to plates for the experimental uses described below.
All mutant and transgenic lines were derived from parental A. thaliana ecotypes Columbia (Col-0), C24, Wassilewskija, or Landsberg erecta (Ler), which were obtained from the Ohio State University Stock Center, Ohio State University, Columbus. Mutant lines included mutants eir1 (auxin transport-deficient and ethylene-insensitive) (C. Lushnig, Whitehead Institute, Cambridge, MA) (12), ein2 [ethylene-insensitive (13)] (J. R. Ecker, University of Pennsylvania, Philadelphia), cbb1 [insensitive to brassinosteroid (14)] (C. Müssig, Max-Planck-Institut, Golm, Germany), gai2 [insensitive to gibberellic acid (15)] (N. P. Harberd, John Innes Centre, Norwich, U.K.), etr1 [ethylene-insensitive (16)] (Ohio State University Stock Center), and cre1 [ethylene-insensitive (17)] (Osaka University, Osaka).
Plant Inoculations.
One day before plant experiments, the bacterial strains were cultured on tryptic soy agar plates as described above and scraped into sterile, distilled water. The liquid suspension culture was diluted with water to yield 109 colony-forming units⋅ml−1 based on optical density and serial dilutions with plate counts. Plastic petri dishes (100 × 15 mm) that contained a center partition (I plates; Fisher Scientific) were prepared with MS solid medium and 2-day-old germinated A. thaliana seedlings (6–10 seedlings per plate) were transferred to one side of the I plates. Treated plants were inoculated with 20 μl of a given bacterial strain or sterile distilled water applied dropwise onto the center of the other side of the I plate that did not contain the seedling. Plates were sealed with parafilm and arranged in a completely randomized design.
Plant Growth Measurements.
Fourteen days after inoculation, total leaf surface area was measured by an integrated digital video image analysis system, Agvision system (AGIMAGE PLUS 1.08; Decangon Devices, Pullman, WA; Panasonic CCTV camera WV-BL200; Secaucus, NJ).
Volatile Collection and Analysis.
Bacterial strains inoculated on MS medium petri dishes were placed in Teflon-framed, sterile glass chambers (18 × 18 × 3 cm) under sterile conditions. The volatile collection chambers contained a sliding-glass top-plate to allow for entry of the cultures. Volatile collection chambers containing inoculated petri dishes were left closed for 24 h to allow for the accumulation of volatile organic compounds (VOCs) in the chamber before flowing air through the collection chambers. Charcoal-purified air was humidified by bubbling through a supersaturated NaCl solution and passed over the bacterial culture at a rate of 1 liter⋅min−1; a sterile cotton plug was placed at the inlet of each chamber. Bacterial volatiles were collected by pulling 0.5 liter⋅min−1 by vacuum through SuperQ adsorbent traps located at the opposite end of the chamber. Illumination was provided by metal halide and sodium lamps for a 16-h-light/8-h-dark photoperiod, with a total light intensity of 700 μmol/m2 per s; temperature was maintained at 28°C. Volatiles were collected at intervals of 24 h over 6 days. Compounds were extracted from filters with 150 μl of dichloromethane, and nonyl acetate (800 ng) was added as an internal standard except for bacterial–volatile extracts to be tested for biological activity.
Extracts were analyzed by capillary gas chromatography (GC) on a 15-m × 0.25-mm (i.d.) fused silica column with a 0.25-μm-thick bonded methyl siloxane (Quadrex, New Haven, CT). Injections were made in the splitless mode for 30 s. The gas chromatograph was operated under the following conditions: injector, 200°C; detector, 210°C; and column oven, 26°C for 3 min and then programmed at a rate of 10°C/min to 180°C and, finally, ramped at a rate of 40°C/min to 200°C for 2 min. The velocity of the carrier gas linear flow was 50 cm/s. Quantification was based on comparison of area under the GC flame ionization on detection peak with the internal standard. For comparisons of the same compound under different treatments, response factors for individual compounds were assumed to be equal. Selected samples also were analyzed by GC-MS on a (ion trap) mass spectrometer (GCQ plus; Thermoquest, Austin, TX) interfaced to a gas chromatograph (Trace GC2000) and operated in the electron impact mode. Injections were made in the splitless mode for 30 s, and samples were analyzed on a 30-m × 0.25-mm (i.d.) DB5 column (J & W Scientific, Folsom, CA) under the same conditions described in GC/FID analysis. The transfer line and ion-source temperature were adjusted to 220°C and 180°C, respectively. The components of the bacterial volatile emission were identified by comparison of GC retention times with those of authentic standards and by comparison of mass spectra with spectra of an Environmental Protection Agency/National Institutes of Health database. For ethylene measurements, slant cultured solid medium was inoculated with 10 μl of bacteria (108 colony-forming units⋅ml−1) and grown in sealed containers for 6 days. One-milliliter air samples then were taken from the headspace and injected directly onto a Photovac 10S Plus gas chromatograph with 10S Plus GC integration software and a CP-Sil 5 CB column (Perkin—Elmer). Oven temperature was held at 30°C, with detector and back-flush flow rates of 15 ml⋅min−1 and analysis time of 85 s. Ethylene peak area was compared with a 2-ppm synthetic standard.
Growth Promotion of VOC Extracts and Synthetic Standards.
Two-day-old germinated A. thaliana Col-0 seedlings were transferred to one side of the I plates. One milliliter of volatile bacterial extract, 2,3-butanediol (99+% purity; Aldrich) diluted in CH2Cl2, or solvent alone was mixed with 0.08 g of lanolin (Sigma), and 20 μl of the resulting suspension was applied to a sterile paper disk (d = 1 cm) (Whatman) on the opposite side of the I plate from the plant seedlings.
Statistical Analysis.
Data were analyzed by ANOVA by using JMP 4.0 software (SAS Institute, Cary, NC). The significance of the effect of PGPR treatments was determined by the magnitude of the F value (P = 0.05). When a significant F test was obtained for treatments, separation of means was accomplished by Fisher's protected least significant difference.
Results
Plant-Growth Promotion by Airborne Bacterial Signal(s).
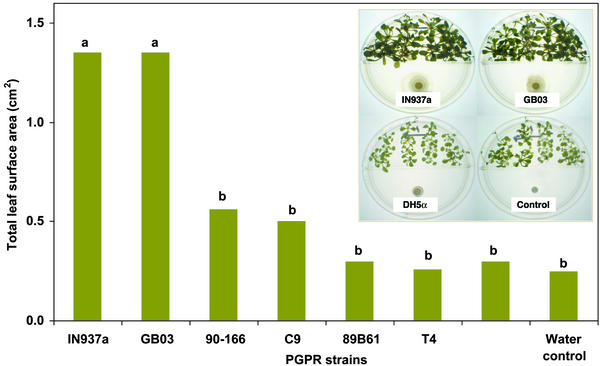
Growth promotion in plants activated by volatile chemicals released from PGPR was tested in the laboratory with divided petri dishes (referred to as I plates) that contain a center partition so that only airborne signals could be transmitted between bacteria and the plant seedlings. Inoculation with two of six strains, GB03 and IN937a, significantly promoted growth compared with the water and DH5α controls (Fig. 1). This selective growth promotion triggered by particular PGPR strains indicated that the release of bacterial VOCs is not the common mechanism for stimulating growth for all rhizobacteria, although VOC growth promotion was observed for both Gram-positive Bacillus spp. (GB03 and IN937a) and Gram-negative E. cloacae strain JM22 (data not shown).
Figure 1.
Quantification of growth promotion in A. thaliana with exposure to airborne chemicals released from six growth-promoting bacterial strains compared with a nongrowth-promoting E. coli strain DH5α and water treatment alone; representative examples of 10-day-old A. thaliana seedlings grown on I plates with airborne exposure to bacteria strains and water treatment are shown in Inset. The I plates were prepared as gnotobiotic systems so that the inoculated bacteria were the only microorganisms present.
Bacterial VOCs Mimic Plant-Growth Promotion by PGPR.
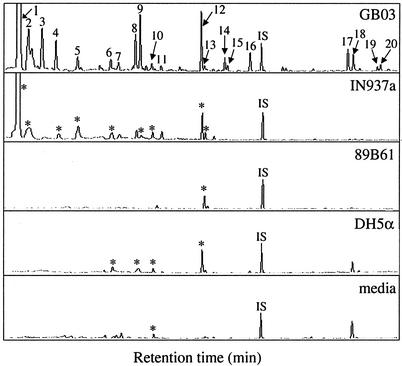
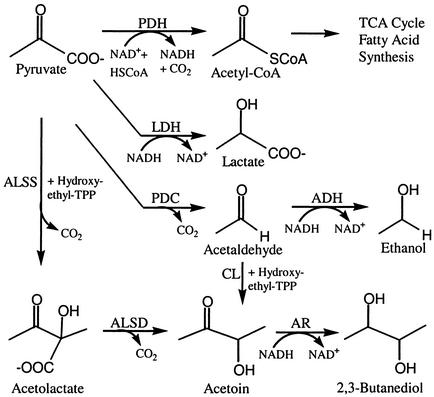
Gas chromatographic analysis of volatiles collected for 24-h intervals revealed consistent differences in the composition of volatile blends released by the growth-promoting bacterial strains GB03 and IN937a (n = 4) compared with the nongrowth-promoting bacterial strain DH5α, 89B61, or MS media alone (Fig. 2). Two compounds, 3-hydroxy-2-butanone [1] and 2,3-butanediol [2], were released consistently from strains GB03 and IN937a, whereas these compounds were not released from strain DH5α, 89B61, or MS medium alone. Over 24-h collection intervals, 3-hydroxy-2-butanone and 2,3-butanediol were two of the most abundant VOCs detected at 12 ± 5 μg [1] and 3.9 ± 0.7 μg [2] for GB03, and 8.8 ± 2.2 μg [1] and 1.9 ± 0.5 μg [2] for IN937a. These volatile alcohols are products of an alternative reductive pathway originating from pyruvate that provides an alternative source of NAD+ under anaerobic conditions (Fig. 3). Indeed, with 3-hydroxy-2-butanone and 2,3-butanediol, the qualitative and quantitative composition of volatile blends emitted by the growth-promoting strains differed significantly from nongrowth-promoting bacteria or medium alone.
Figure 2.
Chromatographic profiles of volatiles from bacteria strains IN937a and GB03, both of which promote growth by the emission of volatile chemicals, compared with a growth-promoting strain that does not trigger promotion by volatile emissions 89B61, a nongrowth-promoting bacterial strain DH5α, and an uninoculated medium control. Compounds positively identified include 3-hydroxy-2-butanone [1], 2,3-butanediol [2], decane [6], tetramethyl pyrazine [9], undecane [10], decanal [13], dodecane [14], 2-undecanone [16], 2-tridecanone [17], and 2-tridecanol [18]; nonyl acetate was added as an internal standard (IS). Asterisks in the lower chromatograms designate compounds that align with numbered peaks above.
Figure 3.
Proposed pathways for anaerobic fermentation in B. subtilis (modified from ref. 11). Enzymes with known coding genes include pyruvate dehydrogenase (PDH), lactate dehydrogenase (LDH), pyruvate decarboxylase (PDC), alcohol dehydrogenase (ADH), acetolactate synthase (ALSS), acetolactate decarboxylase (ALSD), and acetoin reductase (AR).
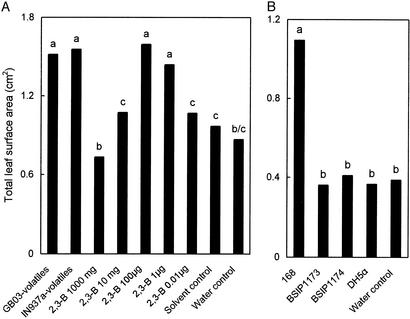
Volatile extracts collected from strains GB03 and IN937a then were tested for biological activity and found to significantly enhance total leaf surface area of A. thaliana compared with the dichloromethane (solvent) control (Fig. 4A). The volatile extract of the nongrowth-promoting bacterium DH5α had no growth-enhancing effect compared with the solvent control.
Figure 4.
Growth promotion of A. thaliana ecotype Col-0 with exposure to extracted bacterial volatiles from growth-promoting (GB03 and IN937a) and nongrowth-promoting (DH5α) bacteria and synthetic 2,3-butanediol (A) and exposure to volatiles released from B. subtilis WT (168) and mutant strains defective in the production of 2,3-butanediol (BSIP1173 and BSIP1174) (B). Different letters indicate significant differences between treatments according to least significant difference at P = 0.05.
The Role of 2,3-Butanediol in Plant-Growth Promotion.
The B. subtilis mutants BSIP1173 and BSIP1174 that do not produce acetoin and 2,3-butanediol because of an insertional knockout of the operon for acetolactate synthase and acetolactate dehydrogenase gene expression were tested directly against the WT strain 168 that is fully functional in acetoin and 2,3-butanediol synthesis. With comparable growth for all three strains on MS medium, the mutant strains BSIP1173 and BSIP 1174 exhibited significantly lower growth promotion of A. thaliana seedlings than the WT strain 168 (Fig. 4B). A dose–response curve with synthetic 2,3-butanediol in the presence of A. thaliana seedlings confirmed the efficacy of this volatile bacterial metabolite in promoting plant growth (Fig. 4A).
Screening Arabidopsis-Signaling Pathway Mutants for Regulatory Control of Growth Promotion.
To probe the mechanism by which bacterial volatiles can enhance plant growth, PGPR strains GB03 and IN937 were tested against a series of A. thaliana mutants defective in specific regulatory pathways. Enhanced total leaf surface area resulted from exposure to both PGPR strains for A. thaliana WTs (Col-0, C-24, and Wassilewskija) and three of four tested mutants (cbb1, gai2, and eir1), thereby negating the essential involvement of the brassinosteroid, gibberellic acid, or ethylene-signaling pathways in the activation of growth promotion by volatile chemicals (Table 1). An exception to this pattern was the cytokinin- and ethylene-insensitive ein 2.5 mutant as well as the cytokinin receptor mutant cre 1, which did not exhibit growth promotion when exposed to strain GB03, suggesting a role for the cytokinin-signaling pathways in growth promotion by bacterial VOC emissions. Because a mutation in auxin transport (eir1) does not necessarily affect auxin action in the leaves, a conclusion about the effect of auxin regulation on growth promotion by PGPR VOCs cannot be made.
Table 1.
Growth promotion response of A. thaliana mutants planted on I plates with airborne exposure to GB03 and IN937a strains
| Strain code | Description | Growth promotion effect
|
|
|---|---|---|---|
| GB03 | IN937a | ||
| Col-0, C24, WS, Ler | Wild types | ++ | ++ |
| ein2 | Cytokinin- and ethylene-insensitive | — | ++ |
| cbb1 | Brassinosteroid-insensitive | ++ | ++ |
| gai2 | Gibberellic acid-insensitive | ++ | ++ |
| eir1 | Auxin-transport-deficient and ethylene-insensitive | ++ | ++ |
| etr1 | Ethylene-insensitive | ++ | ++ |
| cre1 | Cytokinin receptor-deficient | — | ++ |
Symbols indicate that VOCs from the particular bacterial strain did (++) or did not (—) result in significant growth promotion of Arabidopsis seedlings in I plate assays relative to a water (control) treatment.
Discussion
The discovery that bacterial-produced VOCs trigger plant-growth enhancement constitutes an unreported mechanism for the elicitation of plant growth by rhizobacteria. Of the PGPR tested, three of seven strains elicited growth promotion of Arabidopsis seedlings (B. subtilis GB03, B. amyloliquefaciens IN937a, and E. cloacae JM22), suggesting that synthesis of bioactive VOCs is a strain-specific phenomenon. The particular medium in which the bacteria are cultured has been shown to impact the VOC emission profile for certain bacterial strains (18). Our initial screening for plant growth-promoting activity by bacterial VOC emissions was run on a nutrient-rich agar medium (assumed to be nonlimiting in nutrients) that resulted in uniform bacterial growth for all strains tested. Subsequent modifications to the culture medium to reduce VOCs emissions derived from the agar mix did not alter the growth-promoting effects for any of the bacterial strains tested, although it did slow growth for some of the strains (for an example of differential growth, see Fig. 1 Inset). Thus, the reduced bacterial growth in itself was not responsible for the absence or presence of growth promotion observed in the plants tested.
Results of our chemical and biochemical studies indicate that 2,3-butanediol is an essential bacterial component responsible for airborne chemical signaling triggering growth promotion in Arabidopsis based on several experimental results. By comparative analysis of volatile profiles of growth-promoting and non-growth-promoting bacterial strains, release of 2,3-butanediol and acetoin was distinct from other VOCs in that these C4 components were detected exclusively in strains GB03 and IN937a that triggered plant growth promotion by VOC emissions (Fig. 2). The other major GC peaks associated with bacterial VOCs did not show the same regimented emissions from growth-promoting or nongrowth-promoting bacterial strains. Subsequent testing of commercially available 2,3-butanediol established that the exogenous application of this volatile component resulted in a dose-dependent stimulation of plant growth (Fig. 4A). In terms of biologically relevant concentrations, the release rate of ≈10 μg per 24 h for 2,3-butanediol from the growth-promoting strains GB03 and IN937a was within the maximum dose–response range from 1 to 100 μg per experiment observed for the synthetic standard. To minimize the initial burst of 2,3-butanediol released into the air as well as to extend plant exposure to the test compound, a lanolin/2,3-butanediol mixture was used (19). To make comparisons between the amount of airborne VOC that had accumulated in petri dishes with bacterial cultures vs. those treated with an exogenous application of 2,3-butanediol, chambers were allowed to equilibrate for 24 h before VOCs were collected. To confirm the role of 2,3-butanediol in Arabidopsis growth promotion under biological conditions, mutant strains of B. subtilis genetically blocked in the production of 2,3-butanediol were compared with their WT counterparts to examine the effect on plant-growth promotion. In this growth-promotion comparison, knockout mutants of 2,3-butanediol synthesis halted enhanced plant growth whereas WT controls did not (Fig. 4B).
Acetoin and 2,3-butanediol in bacterial systems have been shown to function in shifting glucose catabolism from acidic to neutral products (20). Under low pH and oxygen-limiting conditions, microbes channel pyruvate into the 2,3-butanediol pathway (Fig. 3). Acetolactate synthase catalyzes the condensation of two pyruvate molecules into acetolactate, which is decarboxylated to acetoin. Reduction of acetoin to 2,3-butanediol is thought to contribute to the regulation of the NADH/NAD+ ratio during fermentative metabolism. The specific environmental and cell-life-cycle conditions that regulate acetoin and 2,3-butanediol synthesis are still unclear (20). How 2,3-butanediol may work in concert with other components in the blend of VOCs emitted from rhizobacteria to trigger growth promotion also is open to speculation. The root environment with a low O2 partial pressure as well as the petri dish bioassay systems that we used may result in limited O2 conditions and stimulate this acetoin alternative pathway in the bacteria. Acetoin-forming enzymes also have been identified in tobacco, carrot, maize, and rice cultures, although their function, or even if they normally operate in plants, has yet to be established (20).
The rationale for testing various mutant lines of Arabidopsis was to probe already characterized biosynthetic pathways as potential regulatory sites for triggering growth promotion. Signaling pathways involved in growth promotion and activated by classical plant-growth regulators (21) were tested by using cytokinin-, brassinosteroid-, and gibberellic acid-insensitive mutants as well as auxin-transport-deficient and cytokinin receptor-deficient mutants. A signaling-pathway mutant of ethylene also was examined because of ethylene's role as an airborne signaling molecule in plant development (22) and, specifically, because ethylene recently has been shown to regulate plant growth with reduced elongation and increased thickness in seedling hypocotyls (23). The observation that VOCs from strain IN937a induced growth promotion on all mutant lines tested (Table 1) indicates that the physiological basis for growth promotion was not associated with the gaseous plant regulator ethylene. Further, the physiological basis of growth promotion was not related to one of the common plant-growth regulators, including cytokinins, gibberellic acid, or brassinosteroids.
Although the VOCs from the second PGPR strain, GB03, stimulated growth for several of the mutants, there were exceptions with the cytokinin/ethylene-insensitive mutant ein2 and the cytokinin receptor-deficient mutant cre1. We confirmed the lack of growth promotion of ein2 by VOCs from GB03 in subsequent greenhouse tests (data not shown), where mean foliar fresh weight of ein2 was 33 mg with the water control, 35 mg with GB03, and 106 (statistically significant at P = 0.05) with IN937a. Based on the results with ein2 and cre1, the cytokinin-signaling pathway appears to play some role in growth promotion with exposure to GB03 VOCs.
Histological changes in cell number and/or size can provide information as to whether plant-growth promotion is driven by an increase in cell division and/or regulation of cell expansion. Classical plant growth regulators have been observed to function as activators of cell size and cell numbers. For example, auxin has been identified in the regulation of cell division and differentiation, although its main function is to stimulate cell expansion (21). The expansion process is initiated by the activation of plasma-membrane proton pumps that acidify the cell wall, as well as growth-specific hydrolases that trigger loosening of the cell wall. With subsequent water uptake into the cytosol, a passive increase in cell size occurs. An oxidative burst induced by auxin (resulting in hydroxy radicals) also seems to be involved in cell expansion (24) and might play a role in the signaling events with seedling exposure to 2,3-butanediol. Cytokinins modulate growth by regulating cell division; with the withdrawal of cytokinins in plant tissue, there is an arrest of the cell cycle at the G1 or G2 stage. In the case of plant exposure to ethylene, regulation can occur by both modifications in the rate of cell expansion and cell division. An example of this dual control has been observed in etiolated lupin seedlings with hypocotyl thickening as a result of cell expansion and inhibition of hypocotyls elongation from an inhibition of cell division (23). For our PGPR strains contained within the petri dishes, ethylene levels for strain GB03 (0.9 ± 0.1 ppm) were not significantly different compared with other bacterial treatments (IN937a 1.1 ± 0.1 ppm and DH5α 1.0 ± 0.2 ppm) or the water controls (1.3 ± 0.1 ppm), suggesting that PGPR do not directly affect growth by ethylene degradation. Additional measurements of ethylene emissions at shorter time intervals will establish whether transient changes in ethylene levels occur for the different strains. The mechanism by which volatile components from PGPR promote growth via cell expansion and/or cell division and the interaction of 2,3-butanediol with established plant growth hormones are areas that now can be examined.
From a whole-plant perspective, it remains to be determined whether growth promotion by PGPR VOCs occurs in soil or soil-less medium. It is possible that volatiles produced by PGPR-colonizing roots are generated at sufficient concentrations to trigger plant responses. Indeed, with the low partial pressure of O2 in the root environment, activation of the acetoin pathway is certainly possible.
Acknowledgments
This work was supported by the U.S. Department of Agriculture (Grant 35320-9378) and The Herman Frasch Foundation for Chemical Research.
Abbreviations
- PGPR
plant growth-promoting rhizobacteria
- MS
Murashige and Skoog salt
- VOC
volatile organic compound
References
- 1.Kloepper J W, Zablotowicz R M, Tipping E M, Lifshitz R. In: The Rhizosphere and Plant Growth. Keister K L, Cregan P B, editors. Dordrecht, The Netherlands: Kluwer; 1991. pp. 315–326. [Google Scholar]
- 2.Kloepper J W, Leong J, Teintze M, Schroth M N. Nature. 1980;286:885–886. [Google Scholar]
- 3.Kloepper J W, Schroth M N. Phytopathology. 1981;71:1078–1082. [Google Scholar]
- 4.Loper J E, Schroth M N. Phytopathology. 1986;76:386–389. [Google Scholar]
- 5.Timmusk S, Nicander B, Granhall U, Tillberg E. Soil Biol Biochem. 1999;31:1847–1852. [Google Scholar]
- 6.MacDonald E M S, Powell G K, Regier D A, Glass N L, Roberto F, Kosuge T, Morris R O. Plant Physiol. 1986;82:742–747. doi: 10.1104/pp.82.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glick B R. In: Biochemical and Genetic Mechanisms Used by Plant Growth Promoting Bacteria. Glick B R, Patten C N, Holguin G, Penrose D M, editors. London: Imperial College Press; 1999. pp. 1–13. [Google Scholar]
- 8.Lin W, Okon Y, Hardy R W F. Appl Environ Microbiol. 1983;45:1775–1779. doi: 10.1128/aem.45.6.1775-1779.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer E E. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 10.Farag M A, Paré P W. Phytochemistry. 2002;61:545–554. doi: 10.1016/s0031-9422(02)00240-6. [DOI] [PubMed] [Google Scholar]
- 11.Ramos H C, Hoffmann T, Nedjari H, Presecan-Siedel E, Dreesen O, Glaser P, Jahn D. J Bacteriol. 2000;182:3072–3080. doi: 10.1128/jb.182.11.3072-3080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luschnig C, Gaxiola R A, Grisafi P, Fink G R. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso J M, Hirayama T, Roman G, Nourizadeh S, Ecker J R. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 14.Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Plant J. 1996;9:701–713. [Google Scholar]
- 15.Peng J, Carol P, Richards D E, King K E, Cowling R J, Murphy G P, Harberd N P. Gene Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang C, Kwok S F, Bleecker A B, Meyerowitz E M. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- 18.Fiddaman P J, Rossall S. J Appl Bacteriol. 1994;76:395–405. doi: 10.1111/j.1365-2672.1994.tb01646.x. [DOI] [PubMed] [Google Scholar]
- 19.Kessler A, Baldwin I T. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 20.Forlani G, Mantelli M, Nielsen E. Phytochemistry. 1999;50:255–262. [Google Scholar]
- 21.Trewavas A. In: Biochemistry and Molecular Biology of Plants. Buchanan B B, Gruissen W, Jones R L, editors. Rockville, MD: Am. Soc. Plant Physiologists; 2000. pp. 930–987. [Google Scholar]
- 22.Thomma B P H J, Eggermont K, Broekaert W F, Cammue B P A. Plant Physiol Biochem. 2000;38:421–427. [Google Scholar]
- 23.Nicolas I L, Echeverria M A, Sanchez-Bravo J. Plant Growth Regul. 2001;33:95–105. [Google Scholar]
- 24.Garreton V, Carpinelli J, Jordana X, Holuigue L. Plant Physiol. 2002;130:1516–1526. doi: 10.1104/pp.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]