Abstract
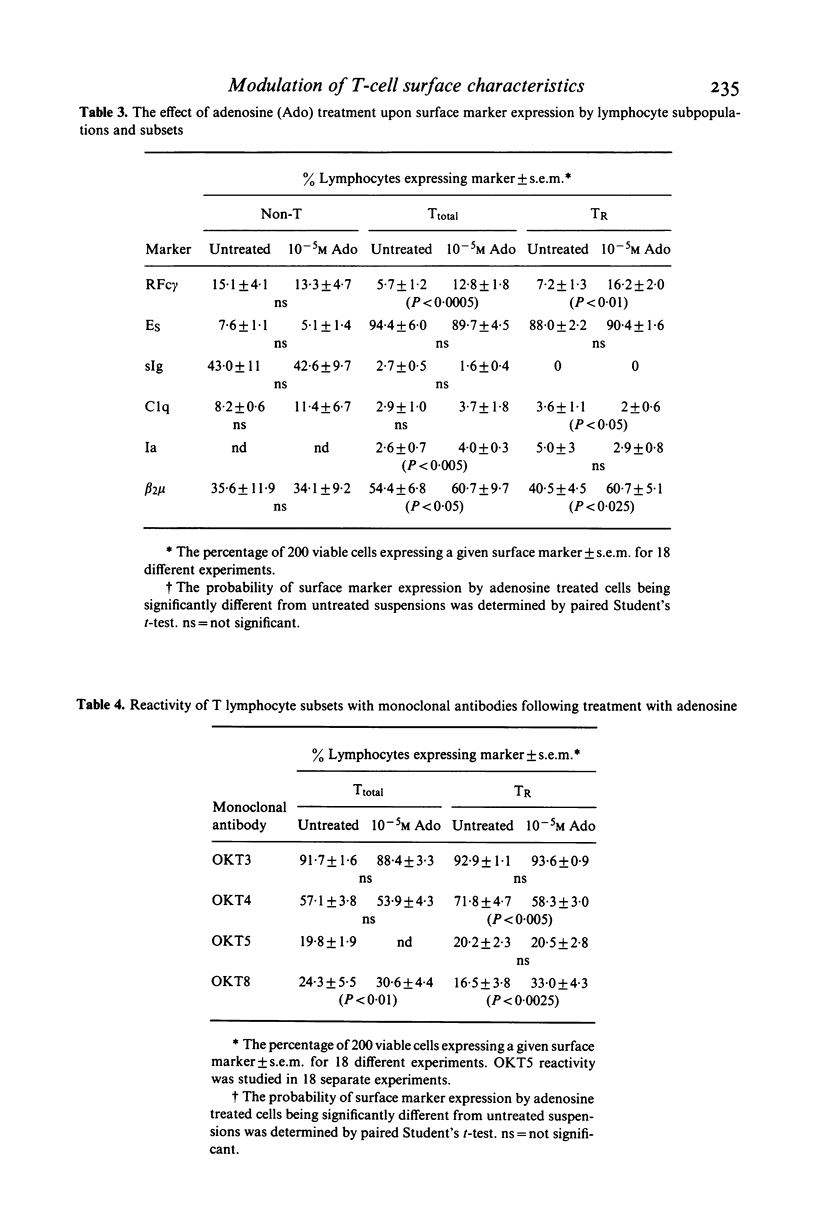
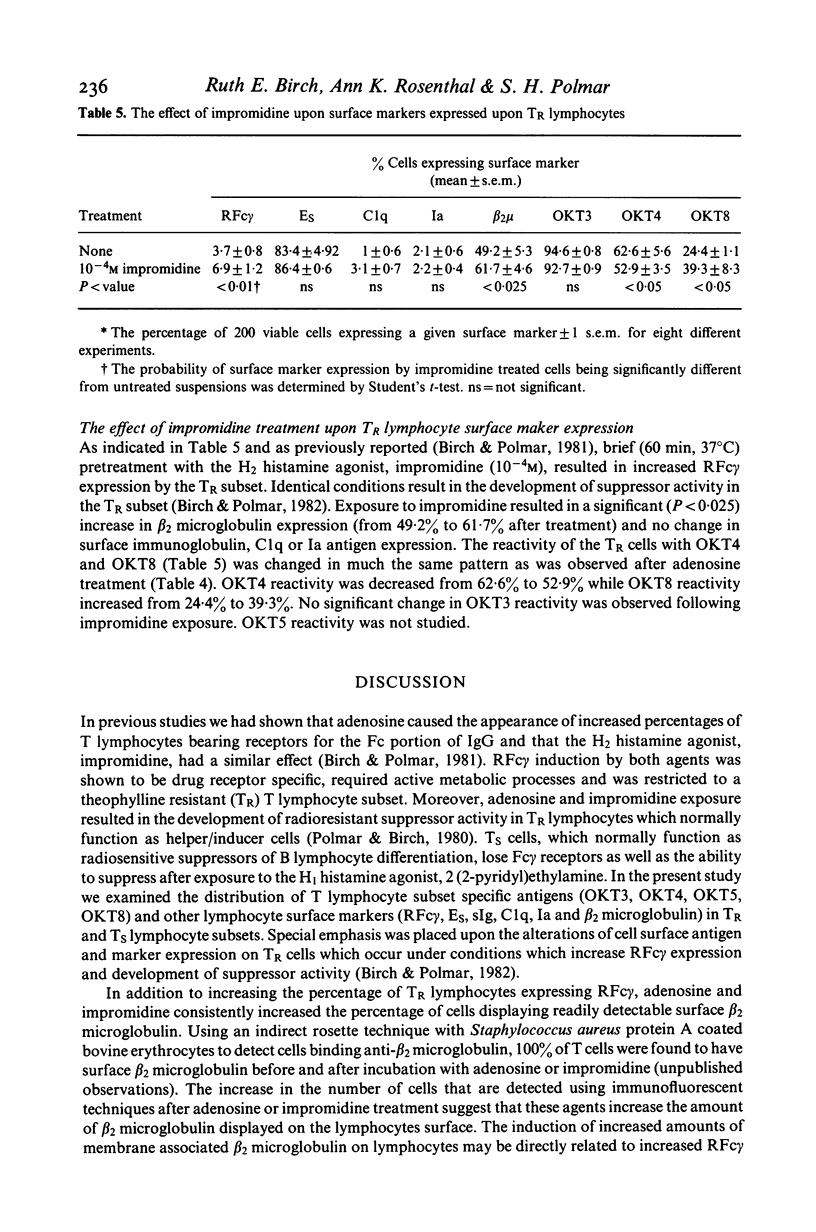
Human peripheral blood lymphocytes were fractionated into non-T lymphocyte, T lymphocyte, theophylline resistant (TR) and theophylline sensitive (Ts) T lymphocyte subpopulations. The proportion of cells bearing surface membrane immunoglobulin (sIg), Clq, Ia antigen, beta 2 microglobulin and T lymphocyte specific antigens detected by monoclonal antibodies OKT3, OKT4, OKT5 and OKT8 was studied using immunofluorescent techniques. Incubation of T lymphocytes or TR lymphocytes with adenosine or impromidine, an H2 histamine agonist, under conditions previously shown to increase Fc gamma receptors and radioresistant suppressor cell activity, was found to increase the proportion of cells expressing readily detectable surface beta 2 microglobulin and the antigen detected by OKT8. Cells expressing OKT4 antigen declined and there was no change in OKT3, OKT5, Ia, Clq, sIg or Es receptor expression. These data indicate that the expression of T lymphocyte Fc gamma receptors, beta 2 microglobulin and the antigens detected by the monoclonal antibodies OKT4 and OKT8 are, at least in part, regulated by agents acting upon adenosine and H2 histamine receptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birch R. E., Bernier G. M., Fanger M. W. Lymphocytes in the rabbit expressing receptors for the Fc portion of rabbit immunoglobulin. J Immunol. 1978 Jun;120(6):1869–1875. [PubMed] [Google Scholar]
- Birch R. E., Fanger M. W., Bernier G. M. Beta 2-microglobulin enhances human lymphocyte surface receptor expression for IgG. J Immunol. 1979 Mar;122(3):997–1001. [PubMed] [Google Scholar]
- Birch R. E., Polmar S. H. Induction of Fc gamma receptors on a subpopulation of human T lymphocytes by adenosine and impromidine, an H2-histamine agonist. Cell Immunol. 1981 Jan 15;57(2):455–467. doi: 10.1016/0008-8749(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Birch R. E., Polmar S. H. Pharmacological modification of immunoregulatory T lymphocytes. I. Effect of adenosine, H1 and H2 histamine agonists upon T lymphocyte regulation of B lymphocyte differentiation in vitro. Clin Exp Immunol. 1982 Apr;48(1):218–230. [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Lazarus H., Penta A. C., Schlossman S. F. Two functionally distinct subpopulations of human T cells that collaborate in the generation of cytotoxic cells responsible for cell-mediated lympholysis. J Immunol. 1978 Apr;120(4):1423–1428. [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limatibul S., Shore A., Dosch H. M., Gelfand E. W. Theophylline modulation of E-rosette formation: an indicator of T-cell maturation. Clin Exp Immunol. 1978 Sep;33(3):503–513. [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Parkman R., Rappeport J., Rosen F. S., Schlossman S. F. Aberrations of suppressor T cells in human graft-versus-host disease. N Engl J Med. 1979 May 10;300(19):1061–1068. doi: 10.1056/NEJM197905103001901. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Rubinstein A., Geha R. S., Strelkauskas A. J., Rosen F. S., Schlossman S. F. Abnormalities of immunoregulatory T cells in disorders of immune function. N Engl J Med. 1979 Nov 8;301(19):1018–1022. doi: 10.1056/NEJM197911083011902. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Strelkauskas A. J., O'Brien C., Schlossman S. F. Phenotypic and functional distinctions between the TH2+ and JRA+ T cell subsets in man. J Immunol. 1979 Jul;123(1):83–86. [PubMed] [Google Scholar]
- Shore A., Dosch H., Gelfand E. W. Induction and separation of antigen-dependent T helper and T suppressor cells in man. Nature. 1978 Aug 10;274(5671):586–587. doi: 10.1038/274586a0. [DOI] [PubMed] [Google Scholar]
- Strelkauskas A. J., Schauf V., Wilson B. S., Chess L., Schlossman S. F. Isolation and characterization of naturally occurring subclasses of human peripheral blood T cells with regulatory functions. J Immunol. 1978 Apr;120(4):1278–1282. [PubMed] [Google Scholar]


