Abstract
The results of this study demonstrate that inhibition of antibody-dependent cell-mediated cytotoxicity (ADCC) of human peripheral blood mononuclear cells (PBMC) by ovalbumin (OA)-IgG anti-OA immune complexes (IC) can be reversed by normal human serum (NHS) or serum from a patient with congenital deficiency of the second component of complement (C2 def-HS) lacking activity of the classical complement (C) pathway. On the other hand, NHS that had been inactivated by heating at 56 degrees C for 30 min (HI-NHS) or NHS depleted of the alternative C pathway activity by absorption with zymosan (Zy-NHS) did not restore the ADCC of IC-blocked PBMC. These results suggest that the alternative pathway of C plays a very important role in the re-establishment of ADCC of PBMC blocked with IC. The recovered activity was susceptible to a new inhibition when re-exposed to IC, demonstrating that the NHS effect depends on the recovery of the functional activity of the receptor for the FC fragment of IgG on PBMC and not on the induction of non-specific cytotoxicity. The ability of NHS to restore the cytolytic potential of IC-inhibited PBMC was dependent on the time of exposure of PBMC to IC before the addition of the unblocking agent (NHS). After prolonged reaction of PBMC with IC, the blocked cells were unable to recover their ADCC activity by incubation with NHS. Unblocking of the Fc receptor of PBMC by C may be a physiological way to prevent permanent impairment of the immune mechanisms that depend on its function in the free state.
Full text
PDF


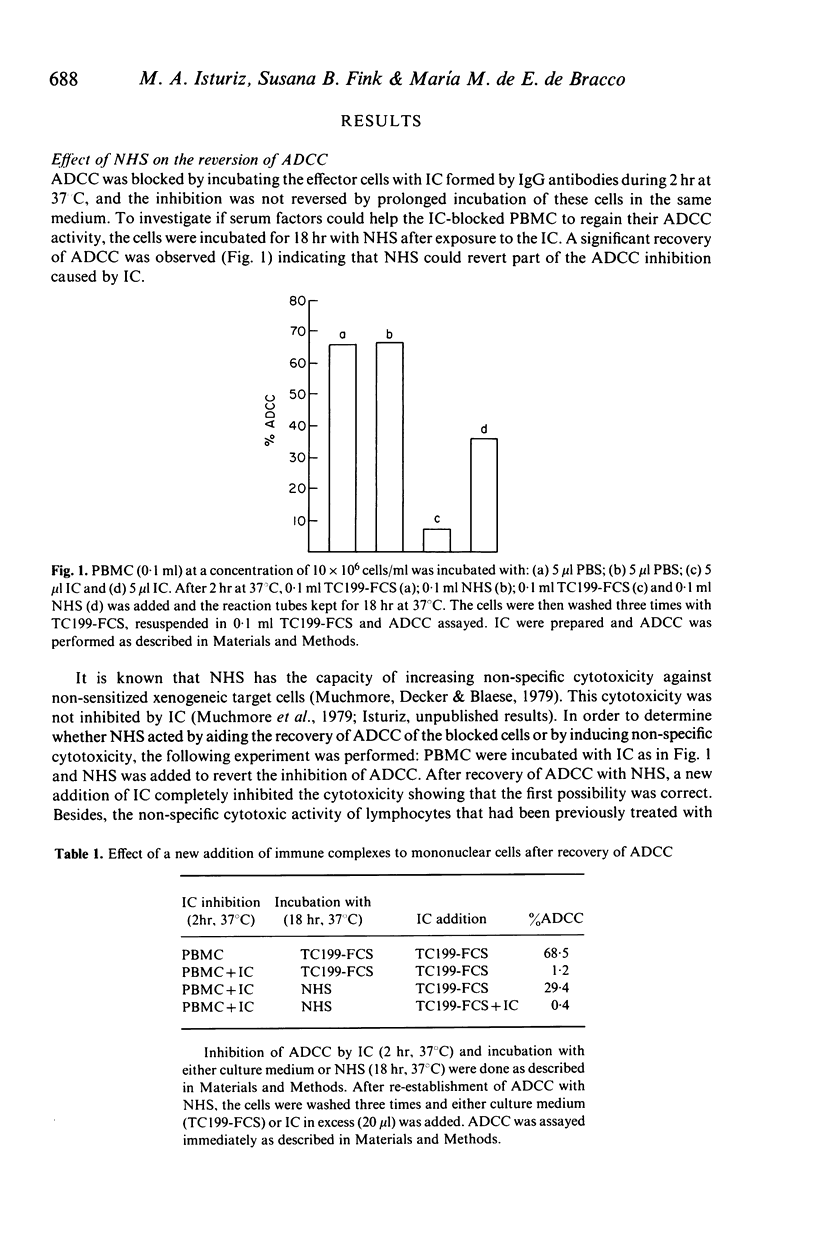
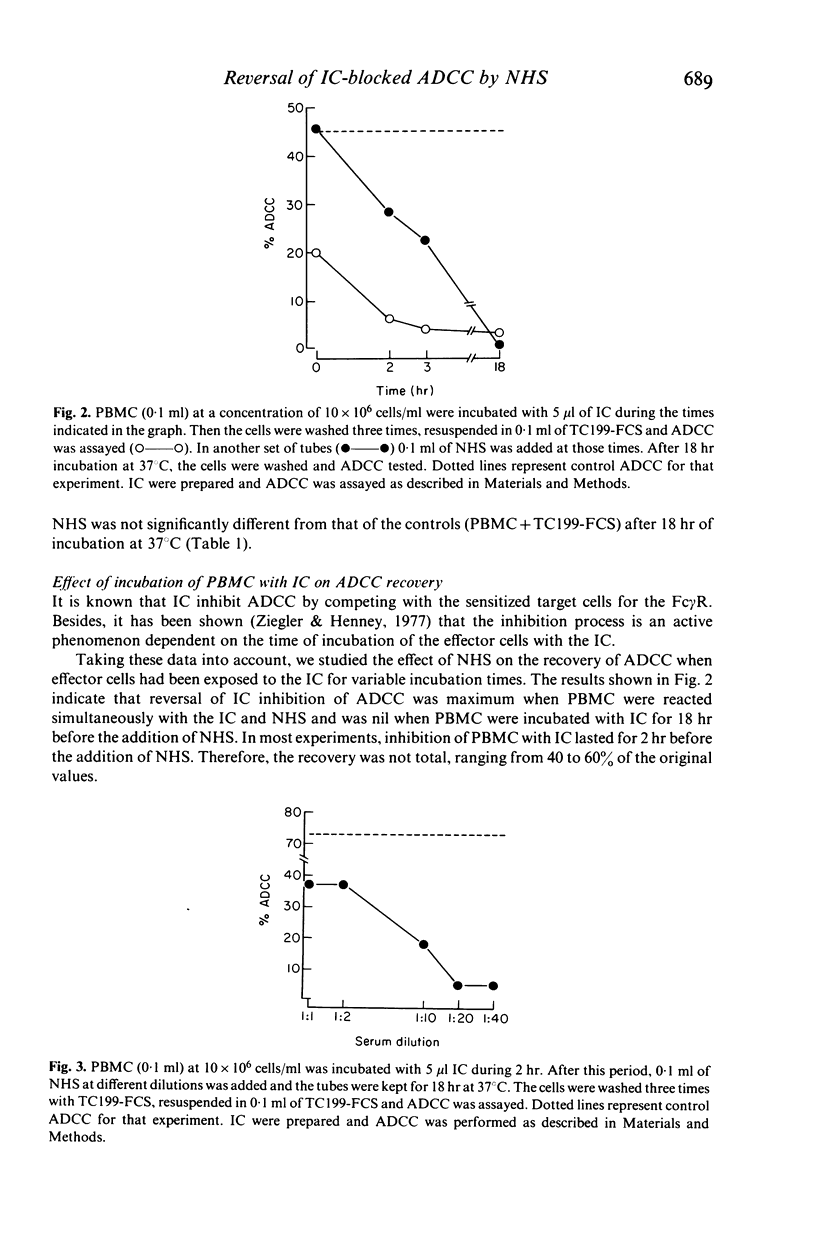



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguado M. T., Perrin L. H., Ramirez R., Miescher P. A., Lambert P. H. Evaluation of alternative pathway and factor B haemolytic activities in patients with systemic lupus erythematosus: correlations with the alternative pathway regulatory proteins. Clin Exp Immunol. 1980 Dec;42(3):495–505. [PMC free article] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. A., Weigle W. O. B-lymphocytes activation by the Fc region of IgG. J Exp Med. 1977 Jul 1;146(1):241–256. doi: 10.1084/jem.146.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch R. E., Fanger M. W., Bernier G. M. Beta 2-microglobulin enhances human lymphocyte surface receptor expression for IgG. J Immunol. 1979 Mar;122(3):997–1001. [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B. Studies of the human lymphocyte receptor for heat-aggregated or antigen-complexed immunoglobulin. J Exp Med. 1974 Aug 1;140(2):508–522. doi: 10.1084/jem.140.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Froland S. S., Wisloff F., Michaelsen T. E. Human lymphocytes with receptors for IgG. A population of cells distinct from T- and B-lymphocytes. Int Arch Allergy Appl Immunol. 1974;47(1):124–138. doi: 10.1159/000231207. [DOI] [PubMed] [Google Scholar]
- Gale R. P., Zighelboim J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol. 1975 Mar;114(3):1047–1051. [PubMed] [Google Scholar]
- Isturiz M. A., de Bracco M. M., Manni J. A. Reaction of human lymphocytes with aggregated IgG columns. Effect on antibody-dependent cytotoxicity and EAC rosette formation. Cell Immunol. 1975 Mar;16(1):82–91. doi: 10.1016/0008-8749(75)90187-2. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Davies A. J. The possible biological significance of Fc receptors on mammalian lymphocytes and tumor cells. Cell. 1974 Oct;3(2):105–112. doi: 10.1016/0092-8674(74)90113-5. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- Lee S. T., Paraskevas F. Cell surface-associated gamma globulins in lymphocytes. IV. Lack of detection of surface globulin on B-cells and acquisition of surface G globulin by T-cells during primary response. J Immunol. 1972 Dec;109(6):1262–1271. [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Saluk P. H., Nussenzweig V. Complement-dependent release of immune complexes from the lymphocyte membrane. J Exp Med. 1973 Sep 1;138(3):495–507. doi: 10.1084/jem.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi Y., Tanimoto K., Yoshinoya S., Mitamura T., Morito T., Nakai H., Horiuchi Y., Umeda T. Detection of Fc-receptor on human renal glomerulus. Clin Immunol Immunopathol. 1978 Jun;10(2):129–135. doi: 10.1016/0090-1229(78)90020-x. [DOI] [PubMed] [Google Scholar]
- Morgan E. L., Walker S. M., Thoman M. L., Weigle W. O. Regulation of the immune response. I. The potentiation of in vivo and in vitro immune responses by Fc fragments. J Exp Med. 1980 Jul 1;152(1):113–123. doi: 10.1084/jem.152.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H. Contactual lysis of antibody-coated chicken erythrocytes by purified lymphocytes. Cell Immunol. 1970 Sep;1(3):300–315. doi: 10.1016/0008-8749(70)90051-1. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Smith J. B. Reversible blocking of antibody-dependent cell-mediated cytotoxicity. Cell Immunol. 1977 May;30(2):353–357. doi: 10.1016/0008-8749(77)90078-8. [DOI] [PubMed] [Google Scholar]
- Thoman M. L., Morgan E. L., Weigle W. O. Fc fragment activation of T lymphocytes. I. Fc fragments trigger Lyt 1+23- T lymphocytes to release a helper T cell-replacing activity. J Immunol. 1981 Feb;126(2):632–635. [PubMed] [Google Scholar]
- Thoman M. L., Morgan E. L., Weigle W. O. Polyclonal activation of murine B lymphocytes by Fe fragments. II. Replacement of T cells by a soluble helper T cell-replacing factor (TRF). J Immunol. 1980 Oct;125(4):1630–1633. [PubMed] [Google Scholar]
- de Bracco M. M., Bianchi C. A., Bianchi O., Stringa S. G. Hereditary complement (C2) deficiency with discoid lupus erythematosus and idiopathic atrophoderma. Int J Dermatol. 1979 Nov;18(9):713–717. doi: 10.1111/j.1365-4362.1979.tb05007.x. [DOI] [PubMed] [Google Scholar]


