Abstract
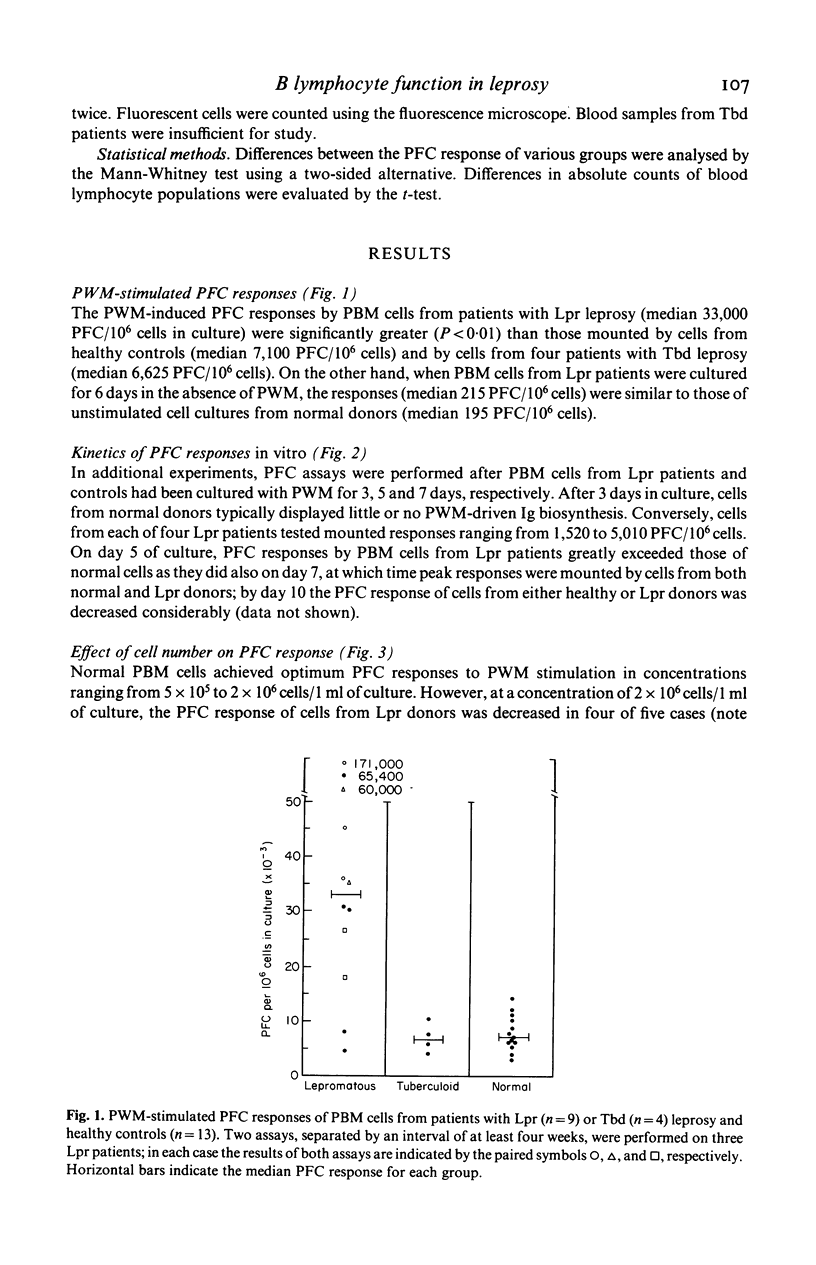
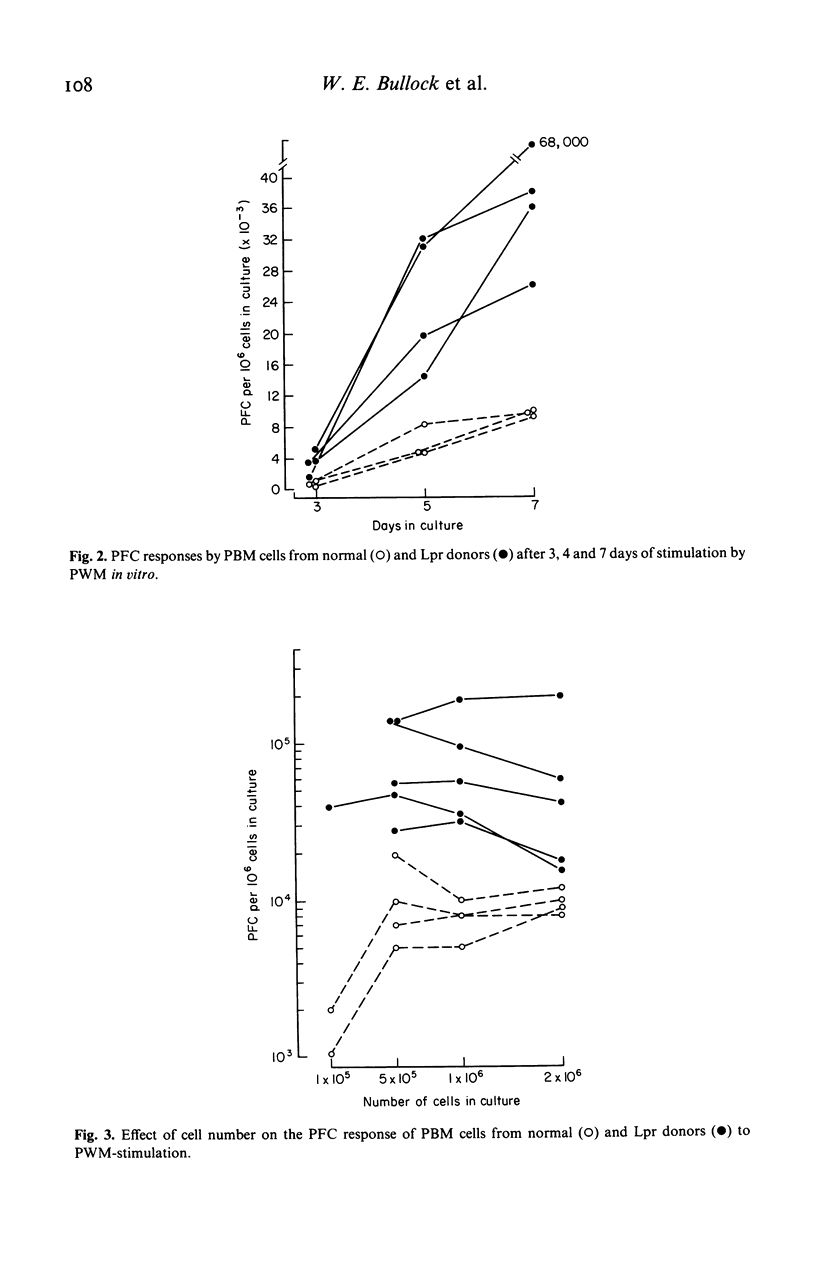
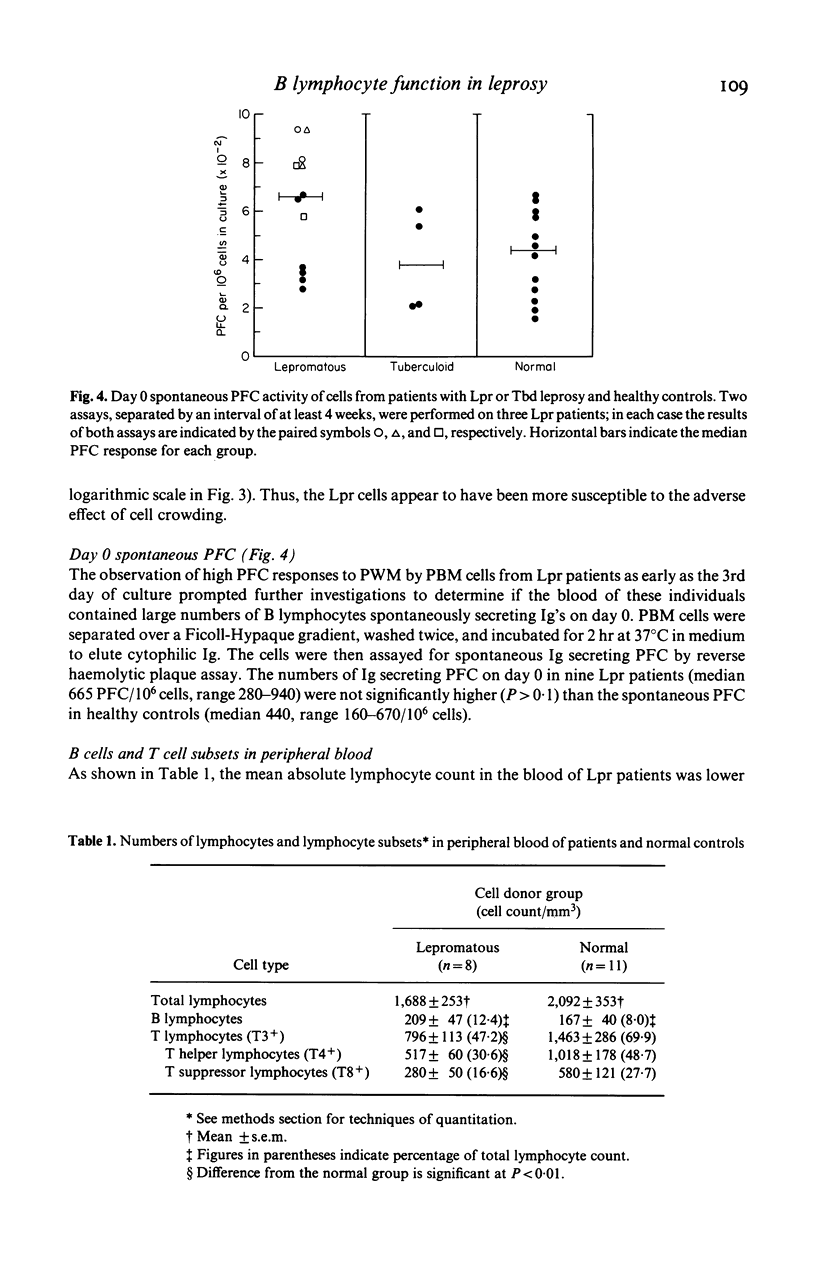
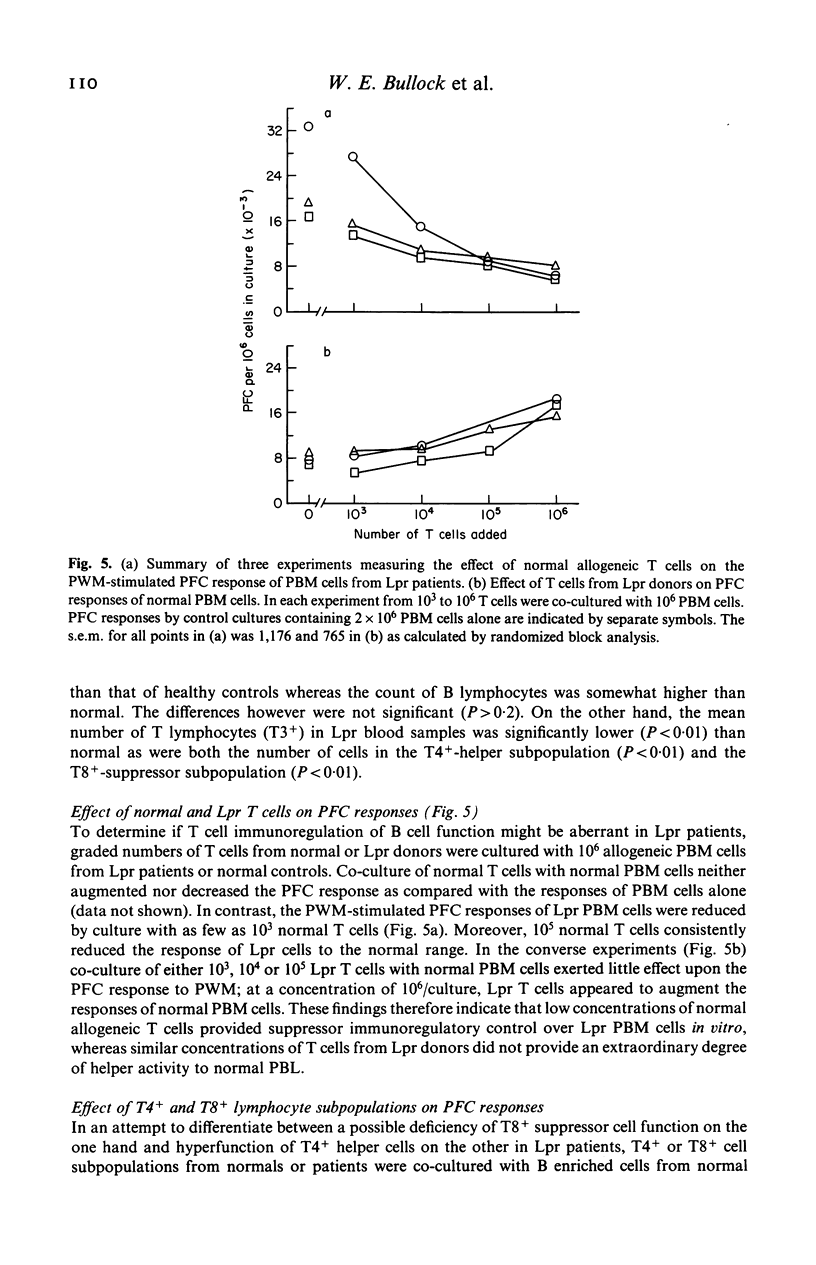
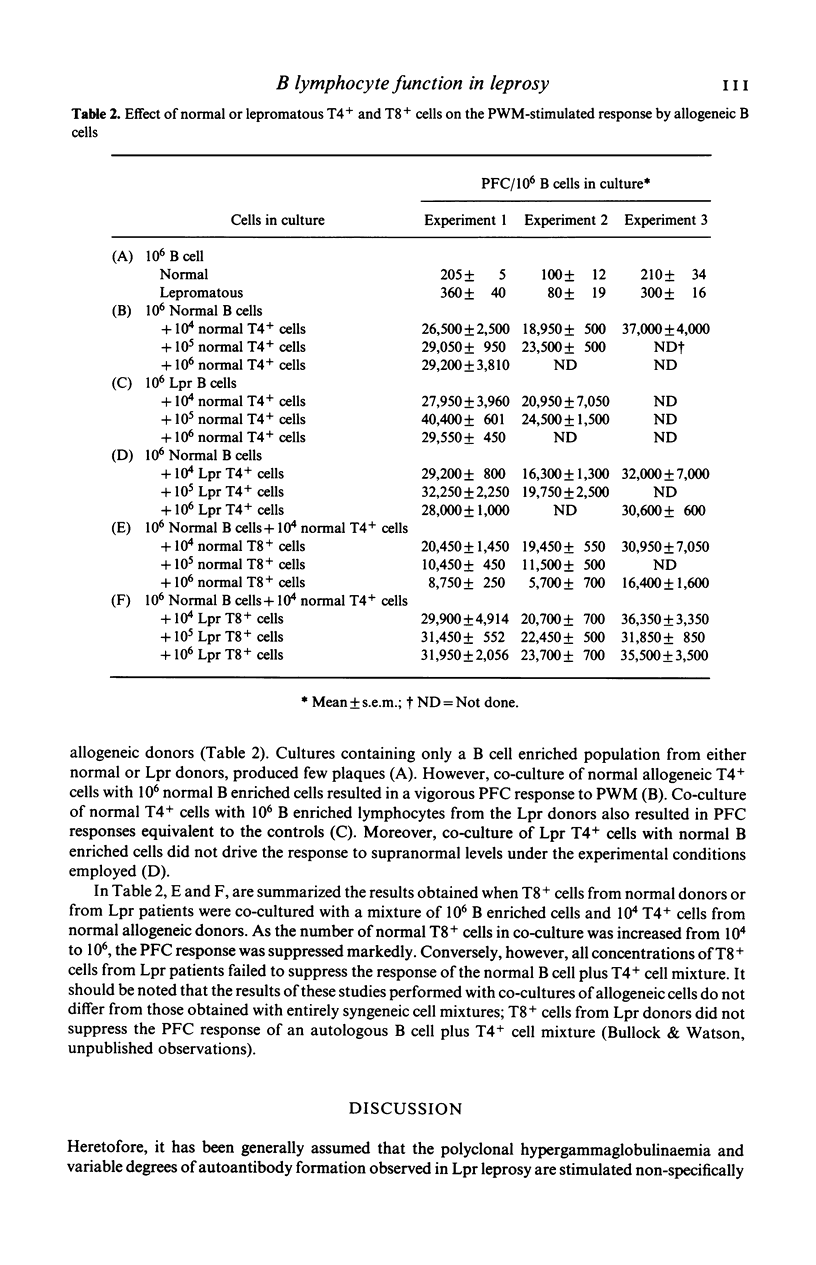
The capacity of peripheral blood mononuclear (PBM) cells from patients with leprosy to generate immunoglobulin-secreting cells in response to pokeweed mitogen (PWM) was evaluated by a reverse haemolytic plaque forming cell (PFC) assay. The PFC responses of PBM cells from patients with lepromatous (Lpr) leprosy were significantly higher (P less than 0.01) than those of PBM cells from normal controls and patients with tuberculoid leprosy. Co-culture of T lymphocytes from normal donors with PBM cells from Lpr patients reduced the PFC response of these cells to the normal range. T4+-helper lymphocytes from Lpr donors did not induce supranormal responses to PWM by normal PBM cells enriched for B lymphocytes. T8+-suppressor lymphocytes from normal donors greatly reduced the response of cultures containing normal allogeneic B cells plus T4+ cells. Conversely, when T8+ cells from Lpr donors were cocultured with normal B cells plus T4+ cells, they failed to suppress the response to PWM. In summary, these studies have demonstrated abnormally high PWM-stimulated PFC responses by B lymphocytes from patients with Lpr leprosy. This aberration, in turn, is associated with a loss of regulatory function by T8+-suppressor cells in Lpr patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Jacobson R. R., Bullock W. E. Decreased suppressor cell activity in disseminated granulomatous infections. Clin Exp Immunol. 1980 Aug;41(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- BUCK A. A., HASENCLEVER H. F. The influence of leprosy on delayed-type skin reactions and serum agglutination titers to Candida albicans. A comparative study of patients with lepromatous and tuberculoid leprosy and controls in Ethiopia. Am J Hyg. 1963 May;77:305–316. doi: 10.1093/oxfordjournals.aje.a120321. [DOI] [PubMed] [Google Scholar]
- Bahr G. M., Rook G. A., Stanford J. L. Inhibition of the proliferative response of peripheral blood lymphocytes to mycobacterial or fungal antigens by co-stimulation with antigens from various mycobacterial species. Immunology. 1981 Nov;44(3):593–598. [PMC free article] [PubMed] [Google Scholar]
- Bjune G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin Exp Immunol. 1979 Jun;36(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Blaese R. M., Grayson J., Steinberg A. D. Increased immunoglobulin-secreting cells in the blood of patients with active systemic lupus erythematosus. Am J Med. 1980 Sep;69(3):345–350. doi: 10.1016/0002-9343(80)90003-0. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E. Leprosy: a model of immunological perturbation in chronic infection. J Infect Dis. 1978 Mar;137(3):341–354. doi: 10.1093/infdis/137.3.341. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Fauci A. S., Whalen G., Burch C. Activation of human B lymphocytes XVI. Cellular requirements, interactions, and immunoregulation of pokeweed mitogen-induced total-immunoglobulin producing plaque-forming cells in peripheral blood. Cell Immunol. 1980 Aug 15;54(1):230–240. doi: 10.1016/0008-8749(80)90204-x. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Abe T., Homma M., Schlossman S. F. Characteristics of anti-T-cell antibodies in systemic lupus erythematosus: evidence for selective reactivity with normal suppressor cells defined by monoclonal antibodies. Clin Immunol Immunopathol. 1980 Aug;16(4):474–484. doi: 10.1016/0090-1229(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F., Schur P. H., Mills J. A., Steinberg A. D. Alterations in immunoregulatory T cell subsets in active systemic lupus erythematosus. J Clin Invest. 1980 Nov;66(5):1171–1174. doi: 10.1172/JCI109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath I., Narayanan R. B., Mehra N. K., Sharma A. K., Gupte M. D. Concanavalin A induced suppressor activity in human leprosy. J Clin Lab Immunol. 1979 Nov;2(4):319–324. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell antibody in systemic lupus erythematosus. Possible mechanism for suppressor-cell dysfunction. J Clin Invest. 1979 Mar;63(3):536–539. doi: 10.1172/JCI109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Touw J., Stoner G. L., Belehu A. Effect of Mycobacterium leprae on lymphocyte proliferation: suppression of mitogen and antigen responses of human peripheral blood mononuclear cells. Clin Exp Immunol. 1980 Sep;41(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Steinberg A. D. A serum inhibitor of immune regulation in patients with systemic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):713–715. doi: 10.1172/JCI109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Nagaoki T., Yokoi T., Mukai M., Uwadana N., Taniguchi N. Regulation of B cell differentiation by T cell subsets defined with monoclonal OKT4 and OKT8 antibodies in human cord blood. J Immunol. 1981 Oct;127(4):1314–1317. [PubMed] [Google Scholar]


