Abstract
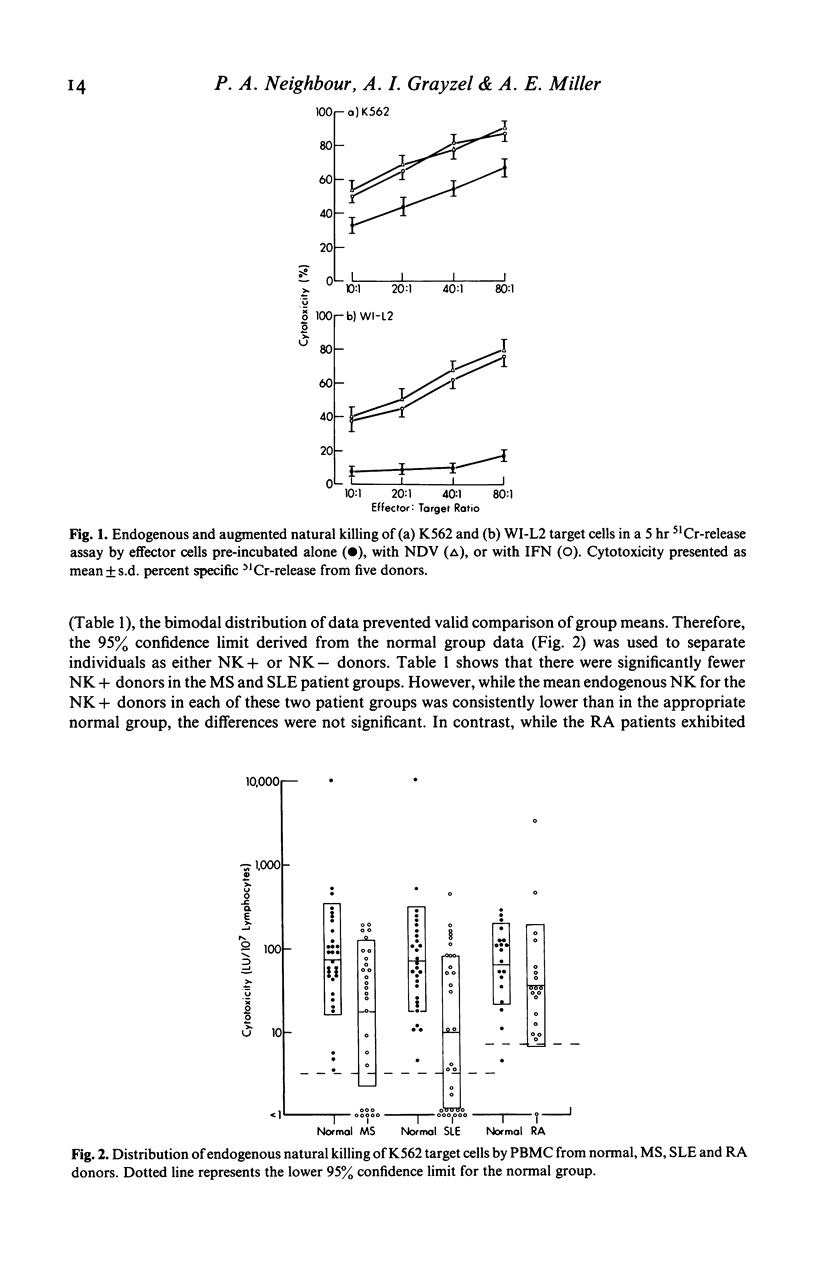
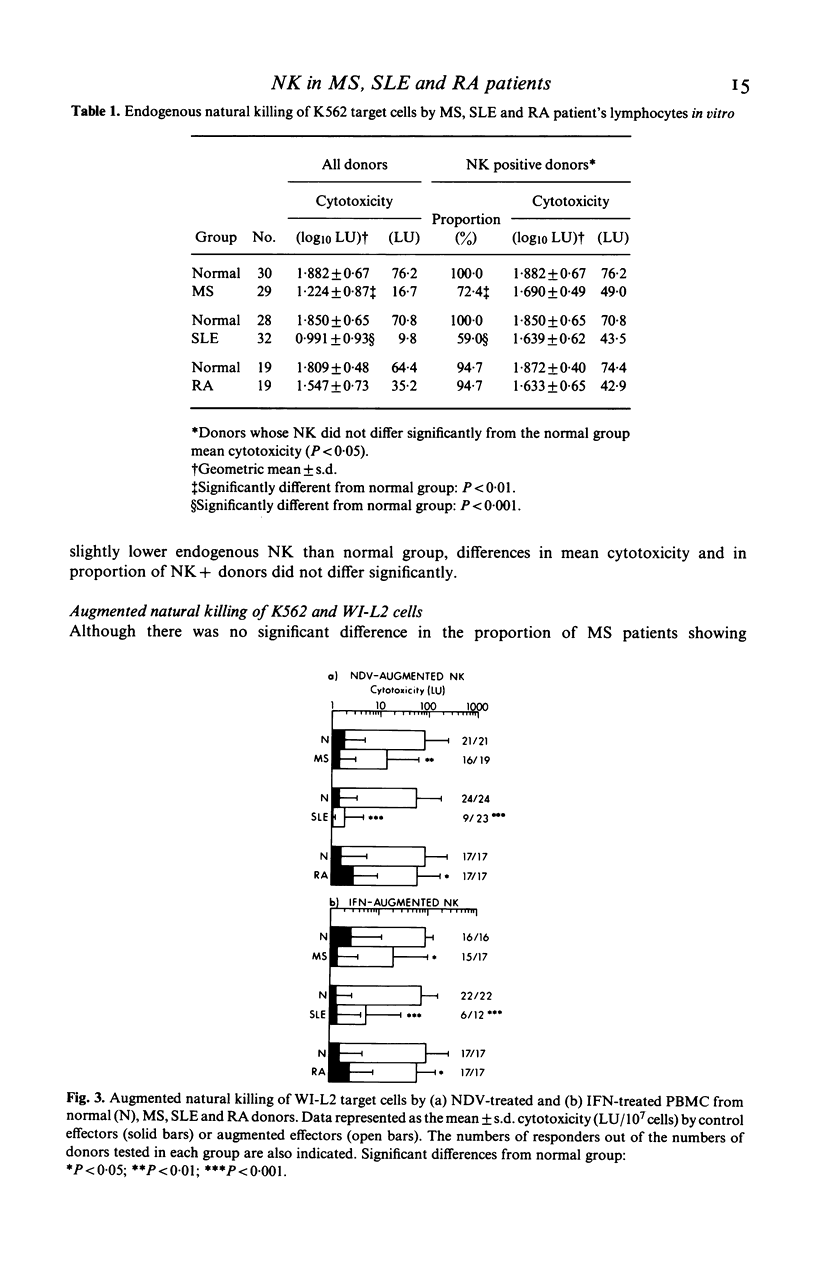
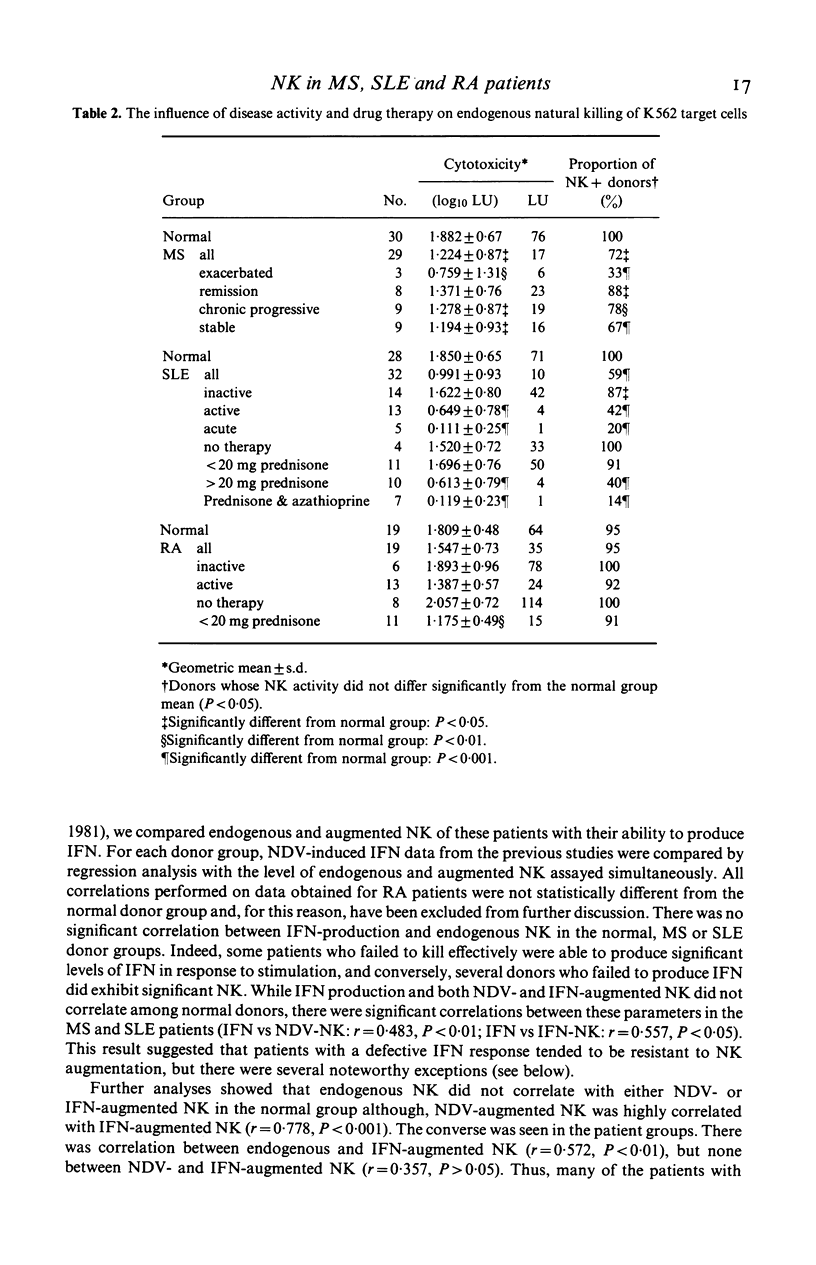
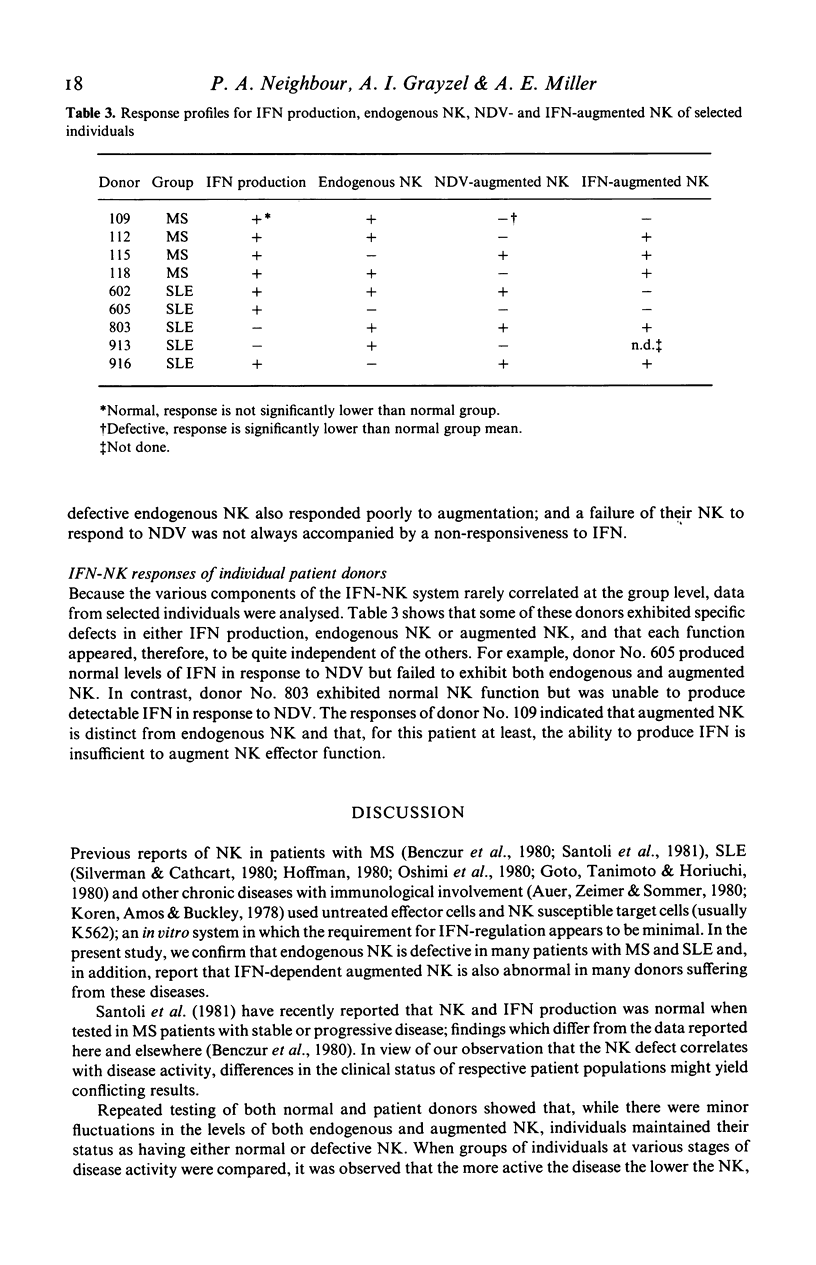
Peripheral blood mononuclear cells (PBMC) of normal human donors are spontaneously cytotoxic for certain tumour-derived and virus-infected target cells. This so-called natural killing (NK) can be augmented by the action of interferons (IFN) and by IFN-inducers. In this study, we have compared both endogenous and augmented NK activity of normal donors with that of patients suffering from either multiple sclerosis (MS), systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA). Endogenous NK was assayed using an NK susceptible target cell (K562), and augmented NK using a target cell (WI-L2) which is lysed only by NK effector cells that have been pre-stimulated by IFN or IFN-inducers. While NK function appeared normal in RA patients, this study confirms previous reports of defective endogenous NK in many MS and SLE patients. In addition, anomalous IFN-augmented NK was also detected in many patients with these two diseases, indicating that defective NK function cannot always be corrected by IFN treatment in vitro. Analysis of IFN production, endogenous NK and IFN-augmented NK by individual patients with MS or SLE showed the defects in their IFN-NK systems to be highly selective, suggesting that individual components of this system may operate independently.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer I. O., Ziemer E., Sommer H. Immune status in Crohn's disease. V. Decreased in vitro natural killer cell activity in peripheral blood. Clin Exp Immunol. 1980 Oct;42(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- Benczur M., Petrányl G. G., Pálffy G., Varga M., Tálas M., Kotsy B., Földes I., Hollán S. R. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin Exp Immunol. 1980 Mar;39(3):657–662. [PMC free article] [PubMed] [Google Scholar]
- Goto M., Tanimoto K., Horiuchi Y. Natural cell mediated cytotoxicity in systemic lupus erythematosus: suppression by antilymphocyte antibody. Arthritis Rheum. 1980 Nov;23(11):1274–1281. doi: 10.1002/art.1780231108. [DOI] [PubMed] [Google Scholar]
- Hoffman T. Natural killer funciton in systemic lupus erythematosus. Arthritis Rheum. 1980 Jan;23(1):30–35. doi: 10.1002/art.1780230106. [DOI] [PubMed] [Google Scholar]
- Hooks J. J., Moutsopoulos H. M., Geis S. A., Stahl N. I., Decker J. L., Notkins A. L. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979 Jul 5;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Amos D. B., Buckley R. H. Natural killing in immunodeficient patients. J Immunol. 1978 Mar;120(3):796–799. [PubMed] [Google Scholar]
- Kurtzke J. F. Neurologic impairment in multiple sclerosis and the disability status scale. Acta Neurol Scand. 1970;46(4):493–512. doi: 10.1111/j.1600-0404.1970.tb05808.x. [DOI] [PubMed] [Google Scholar]
- Minato N., Reid L., Cantor H., Lengyel P., Bloom B. R. Mode of regulation of natural killer cell activity by interferon. J Exp Med. 1980 Jul 1;152(1):124–137. doi: 10.1084/jem.152.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Mingari M. C., Santoli D., Perlmann P., Moretta L. Human T-lymphocyte subpopulations: alterations in systemic lupus erythematosus. Scand J Immunol. 1979;10(3):223–228. doi: 10.1111/j.1365-3083.1979.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Abe T., Homma M., Schlossman S. F. Characteristics of anti-T-cell antibodies in systemic lupus erythematosus: evidence for selective reactivity with normal suppressor cells defined by monoclonal antibodies. Clin Immunol Immunopathol. 1980 Aug;16(4):474–484. doi: 10.1016/0090-1229(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Neighbour P. A., Bloom B. R. Absence of virus-induced lymphocyte suppression and interferon production in multiple sclerosis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):476–480. doi: 10.1073/pnas.76.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbour P. A., Grayzel A. I. Interferon production of vitro by leucocytes from patients with systemic lupus erythematosus and rheumatoid arthritis. Clin Exp Immunol. 1981 Sep;45(3):576–582. [PMC free article] [PubMed] [Google Scholar]
- Neighbour P. A., Miller A. E., Bloom B. R. Interferon responses of leukocytes in multiple sclerosis. Neurology. 1981 May;31(5):561–566. doi: 10.1212/wnl.31.5.561. [DOI] [PubMed] [Google Scholar]
- Oshimi K., Gonda N., Sumiya M., Kano S. Effects of corticosteroids on natural killer cell activity in systemic lupus erythematosus. Clin Exp Immunol. 1980 Apr;40(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Reinherz E. L., Weiner H. L., Hauser S. L., Cohen J. A., Distaso J. A., Schlossman S. F. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980 Jul 17;303(3):125–129. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Saksela E., Timonen T., Cantell K. Human natural killer cell activity is augmented by interferon via recruitment of 'pre-NK' cells. Scand J Immunol. 1979;10(3):257–266. doi: 10.1111/j.1365-3083.1979.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Santoli D., Hall W., Kastrukoff L., Lisak R. P., Perussia B., Trinchieri G., Koprowski H. Cytotoxic activity and interferon production by lymphocytes from patients with multiple sclerosis. J Immunol. 1981 Apr;126(4):1274–1278. [PubMed] [Google Scholar]
- Santoli D., Moretta L., Lisak R., Gilden D., Koprowski H. Imbalances in T cell subpopulations in multiple sclerosis patients. J Immunol. 1978 Apr;120(4):1369–1371. [PubMed] [Google Scholar]
- Silva A., Bonavida B., Targan S. Mode of action of interferon-mediated modulation of natural killer cytotoxic activity: recruitment of pre-NK cells and enhanced kinetics of lysis. J Immunol. 1980 Aug;125(2):479–484. [PubMed] [Google Scholar]
- Silverman S. L., Cathcart E. S. Natural killing in systemic lupus erythematosus: inhibitory effects of serum. Clin Immunol Immunopathol. 1980 Oct;17(2):219–226. doi: 10.1016/0090-1229(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]



