Abstract
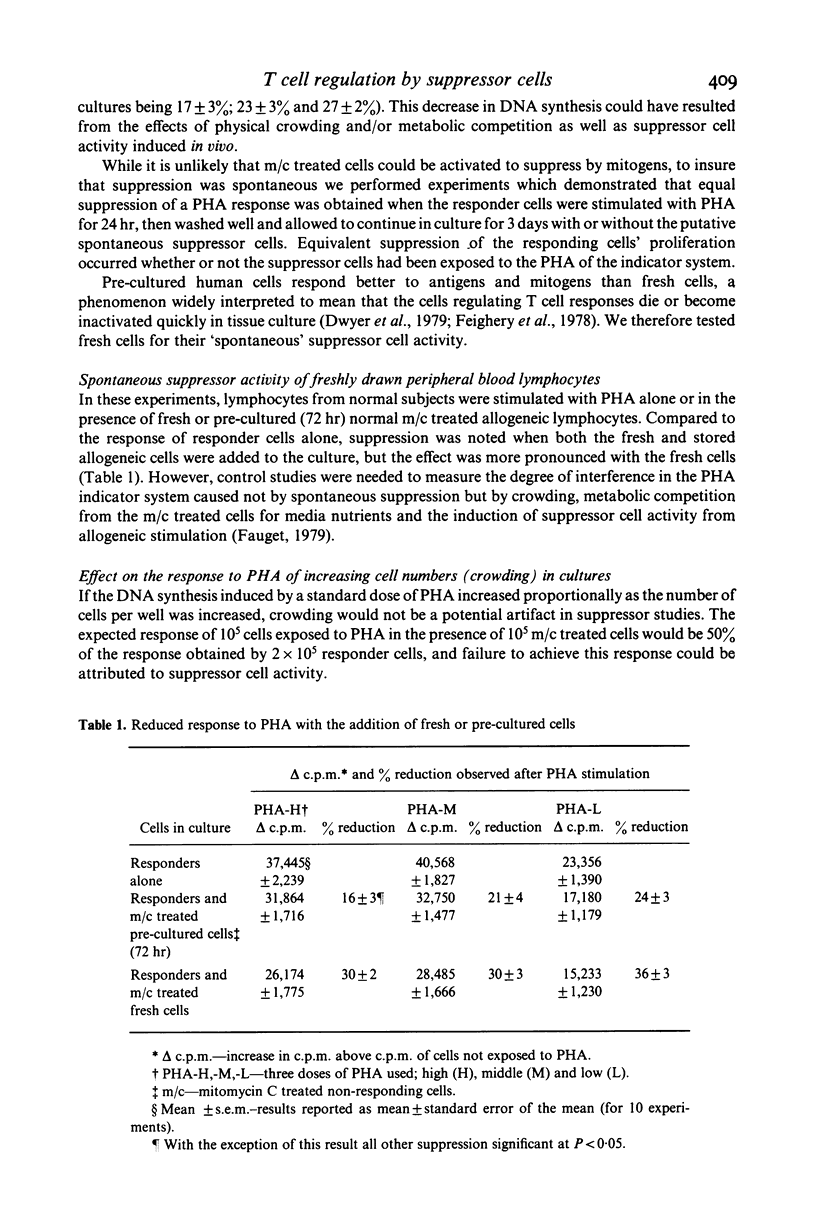
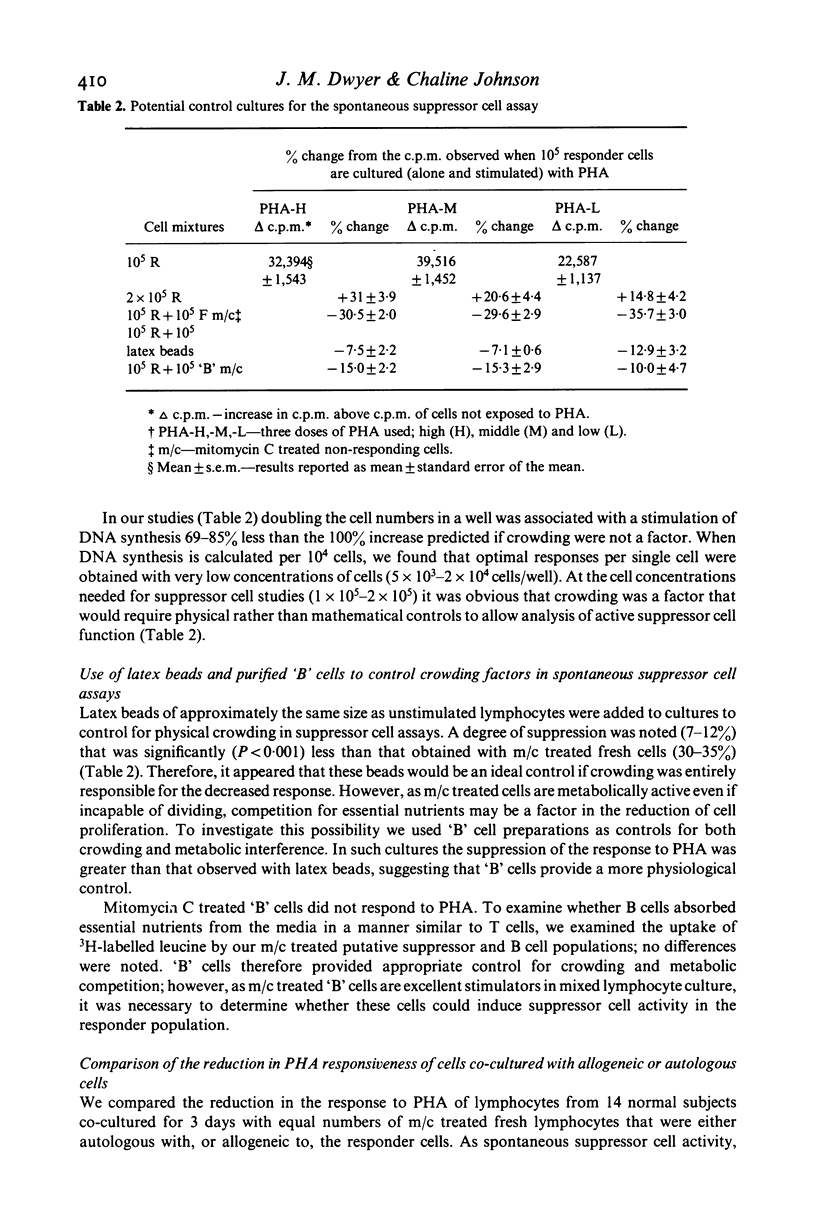
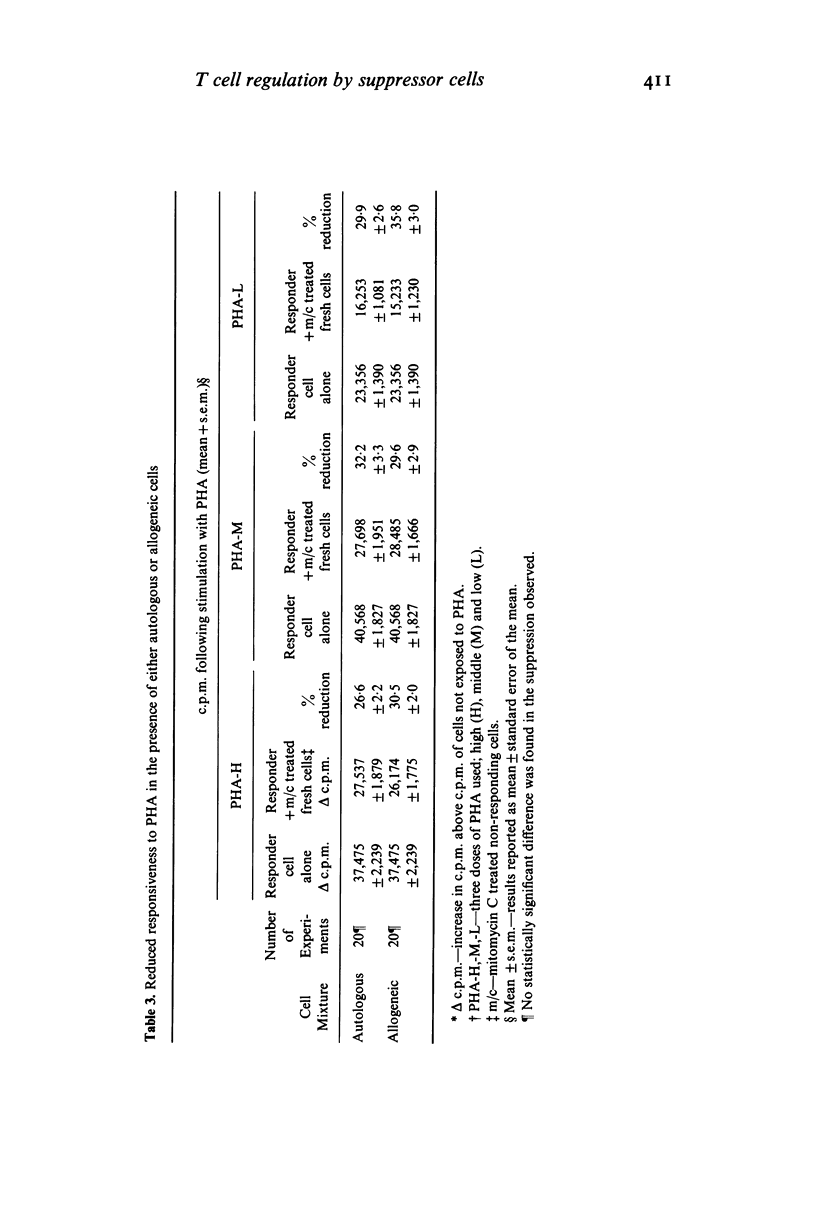
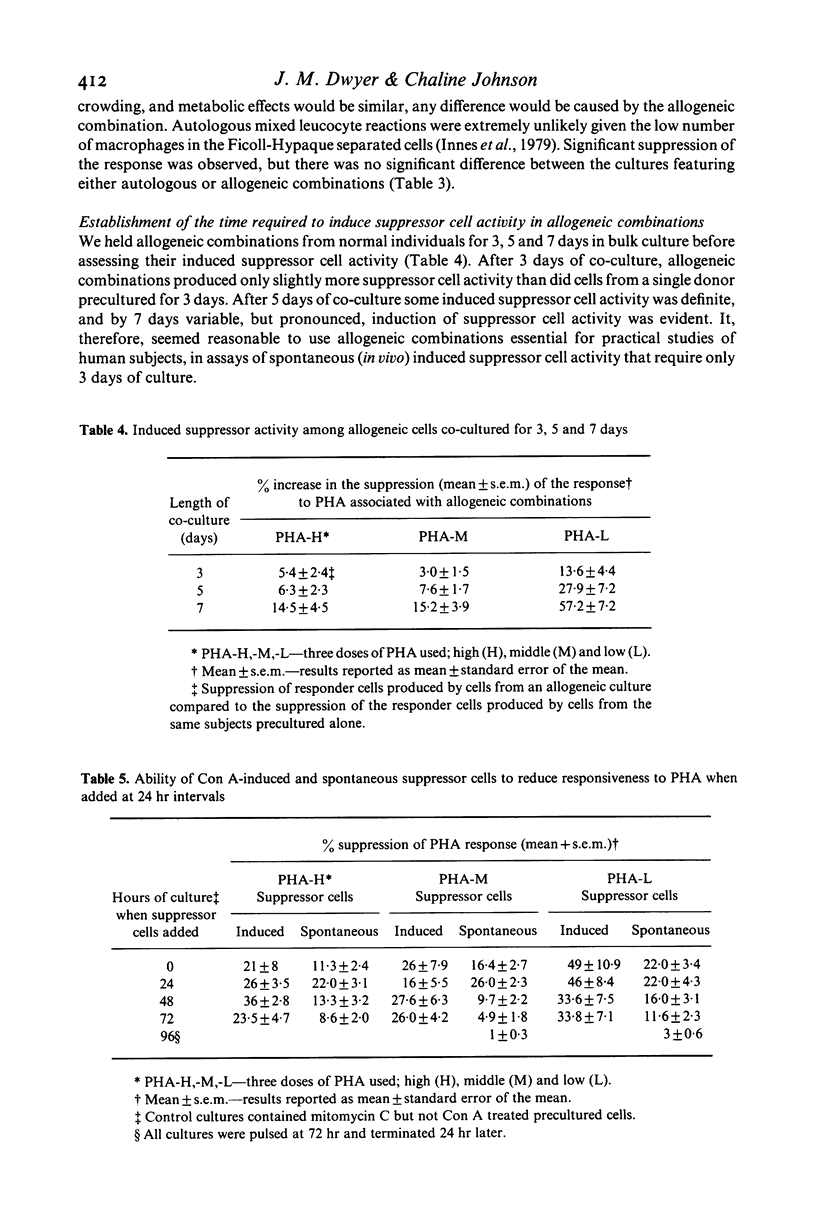
In studying T cell regulation, peripheral blood mononuclear cells from normal subjects were examined for 'spontaneous', rather than mitogen-induced, suppressor cell activity. Normal blood leucocytes from 30 subjects included a subpopulation of cells capable of suppressing the response of lymphocytes to the T cell mitogen phytohaemagglutinin by 21-35%. The indicator system for these studies consisted of fresh normal lymphocytes stimulated by three concentrations of PHA in the presence or absence of normal but mitomycin C treated peripheral blood lymphocytes. To measure accurately the spontaneous suppressor cell activity, additional cultures were needed to control for the suppressive effects of crowding and metabolic competition. Allogeneic, cryopreserved 'B' cell enriched populations, supplied satisfactory control cells for this purpose. While allogeneic culture systems could induce significant suppressor cell activity after 7 days of co-culture, they could not induce this activity in the 3 days required to assay spontaneous suppressor cell effects. In developing this assay we noted that (a) crowding became a factor in the cellular response to mitogens with concentrations higher than 2 X 10(4) cells/well, (b) spontaneous suppressor cell activity decreased rapidly once cells were placed in culture and (c) both spontaneous and concanavalin A (Con A) activated suppressor cells could significantly reduce the response to PHA even when added to cultures established with mitogens 72 hr earlier. The ability to measure spontaneous suppressor cell activity in vitro will allow more physiological studies of the membrane markers and functional characteristics of these cells than is possible in conventional studies utilizing Con A. In addition, this assay allows the detection of enhanced in vivo activity of suppressor cells not easily detected in assays relying on mitogen induction of suppression. Such increased activity is thought to be an important factor in the pathogenesis of a number of human diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Goodgame R. W. Immune responses during human schistosomiasis mansoni. IV. Induction of suppressor cell activity by schistosome antigen preparations and concanavalin A. J Immunol. 1978 Apr;120(4):1225–1232. [PubMed] [Google Scholar]
- Dosch H. M., Gelfand E. W. Functional differentiation of B lymphocytes in agammaglobulinemia. III. Characterization of spontaneous suppressor cell activity. J Immunol. 1978 Nov;121(5):2097–2105. [PubMed] [Google Scholar]
- Faguet G. B. Mechanisms of lymphocyte activation: the role of suppressor cells in the proliferative responses of chronic lymphatic leukemia lymphocytes. J Clin Invest. 1979 Jan;63(1):67–74. doi: 10.1172/JCI109280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Knight S. C. Help and suppression by lymphoid cells as a function of cellular concentration. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3507–3510. doi: 10.1073/pnas.76.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighery C., Whelan C. A., Weir D. G., Greally J. F. In vitro studies of suppressor cell function in human peripheral blood mononuclear cells. Clin Exp Immunol. 1978 Jun;32(3):459–465. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity IV. Antigen-specific suppressor cells for delayed-type hypersensitivity induced by lipopolysaccharide and sheep erythrocytes in mice. Eur J Immunol. 1979 Feb;9(2):101–106. doi: 10.1002/eji.1830090202. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., DeHoratius R., Israel H., Peake G. T., Messner R. P. Suppressor cell function in sarcoidosis. Ann Intern Med. 1979 Feb;90(2):169–173. doi: 10.7326/0003-4819-90-2-169. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. V. Kinetics and mechanisms of suppression of plaque-forming cell responses by concanavalin A-generated suppressor cells. J Immunol. 1978 Mar;120(3):700–708. [PubMed] [Google Scholar]
- Herr H. W. Suppressor cells in the pelvic lymph nodes regional to bladder cancer. J Surg Oncol. 1979;11(4):289–293. doi: 10.1002/jso.2930110403. [DOI] [PubMed] [Google Scholar]
- Innes J. B., Kuntz M. M., Kim Y. T., Weksler M. E. Induction of suppressor activity in the autologous mixed lymphocyte reaction and in cultures with concanavalin A. J Clin Invest. 1979 Dec;64(6):1608–1613. doi: 10.1172/JCI109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer R. S., Clough J. D., Frank S., Sundeen J. T. Suppressor cell function defect in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1979 Nov;14(3):327–333. doi: 10.1016/0090-1229(79)90158-2. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Ginsburg W. W., Finkelman F. D., Ziff M. Control of human B lymphocyte responsiveness: enhanced suppressor T cell activity after in vitro incubation. J Immunol. 1978 Mar;120(3):902–910. [PubMed] [Google Scholar]
- Lobo P. I., Spencer C. E. Inhibition of humoral and cell-mediated immune responses in man by distinct suppressor cell systems. J Clin Invest. 1979 Jun;63(6):1157–1163. doi: 10.1172/JCI109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Miller C. L., Baker C. C. Changes in lymphocyte activity after thermal injury. The role of suppressor cells. J Clin Invest. 1979 Feb;63(2):202–210. doi: 10.1172/JCI109290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble B., Parker D., Scheper R. J., Turk J. L. The relation between B-cell stimulation and delayed hypersensitivity. The effect of cyclophosphamide pretreatment on antibody production. Immunology. 1977 Jun;32(6):885–891. [PMC free article] [PubMed] [Google Scholar]
- Patt Y. Z., Hersh E. M., Dicke K., Spitzer G., Mavligit G. M. Suppressor cell activity in cancer patients: a possible role for thymic hormones. Cancer Treat Rep. 1978 Nov;62(11):1763–1768. [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Saxon A., Stevens R. H. Suppression of immunoglobulin production in normal human blood: characterization of the cells responsible and mediation by a soluble T lymphocyte-derived factor. Clin Immunol Immunopathol. 1978 Aug;10(4):427–437. doi: 10.1016/0090-1229(78)90155-1. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Dupuy J. M. Suppressor activity of lymphocytes from patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1978 Oct;11(2):125–130. doi: 10.1016/0090-1229(78)90037-5. [DOI] [PubMed] [Google Scholar]
- Tosato G., Magrath I., Koski I., Dooley N., Blaese M. Activation of suppressor T cells during Epstein-Barr-virus-induced infectious mononucleosis. N Engl J Med. 1979 Nov 22;301(21):1133–1137. doi: 10.1056/NEJM197911223012101. [DOI] [PubMed] [Google Scholar]
- Tse H. Y., Dutton R. W. Separation of helper and suppressor T lymphocytes. III. Positive and negative effects of mixed lymphocyte reaction-activated T cells. J Immunol. 1978 Apr;120(4):1149–1152. [PubMed] [Google Scholar]
- UytdeHaag F., Heynen C. J., Ballieux R. E. Induction of antigen-specific human suppressor T lymphocytes in vitro. Nature. 1978 Feb 9;271(5645):556–557. doi: 10.1038/271556a0. [DOI] [PubMed] [Google Scholar]



