Abstract
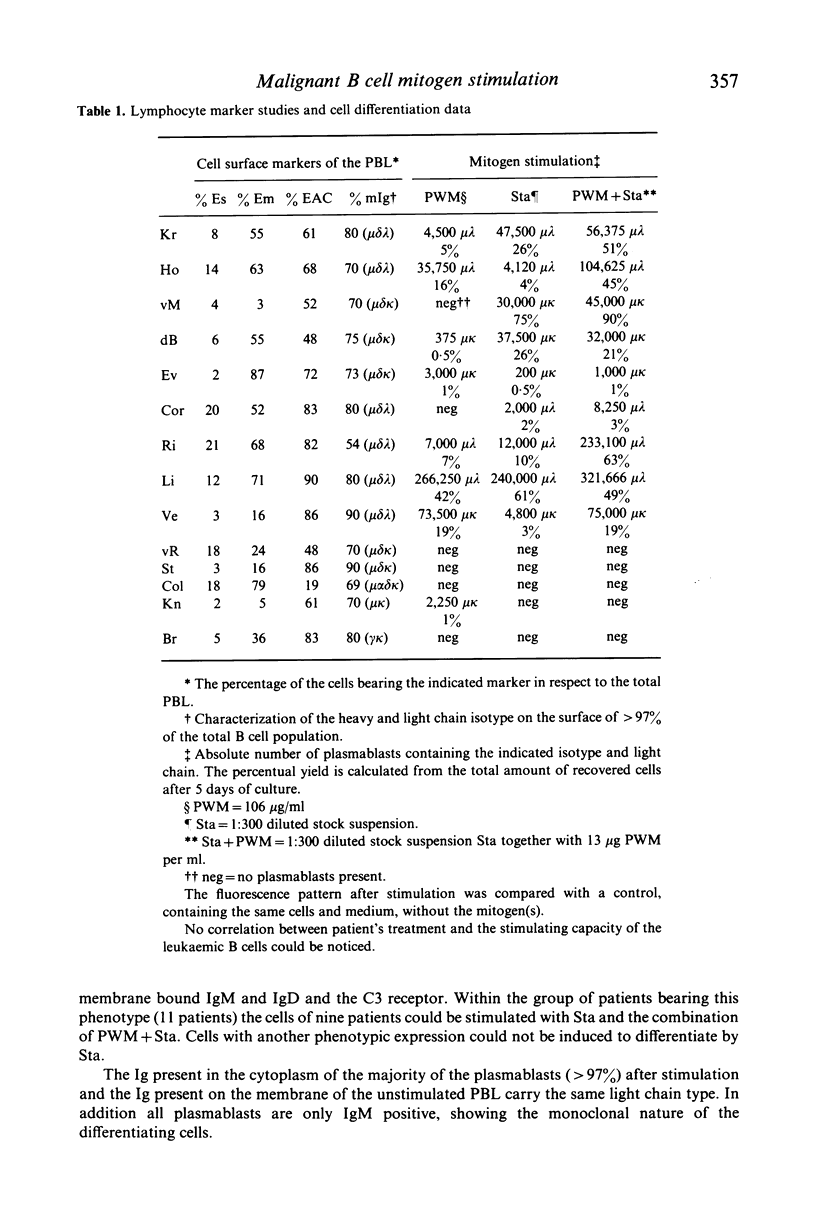
Peripheral blood lymphocytes (PBL) from 14 patients with chronic lymphocytic leukaemia (CLL) were stimulated with pokeweed mitogen (PWM), formalinized Staphylococcus aureus (Sta) and a combination of both mitogens. The leukaemic B cells were characterized by rosetting techniques (using mouse erythrocytes and complement coated erythrocytes) and immunofluorescence for membrane bound immunoglobulin (mIg). No clear correlation between phenotype and the reactivity with PWM could be found. Results of stimulation with Sta however, indicate that lymphocytes carrying membrane bound IgM (mIgM) and IgD (mIgD) and the receptor of the third complement component (C3R) can be induced to differentiate into immunoglobulin (Ig) containing cells. Addition of PWM to these cultures often enhanced this response. Some leukaemic B cells are able to differentiate after challenge with the appropriate stimulus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bona C., Damais C., Dimitriu A., Chedid L., Ciorbaru R., Adam A., Petit J. F., Lederer E., Rosselet J. P. Mitogenic effect of a water-soluble extract of Nocardia opaca: a comparative study with some bacterial adjuvants on spleen and peripheral lymphocytes of four mammalian species. J Immunol. 1974 Jun;112(6):2028–2035. [PubMed] [Google Scholar]
- Chiorazzi N., Fu S. M., Montazeri G., Kunkel H. G., Rai K., Gee T. T cell helper defect in patients with chronic lymphocytic leukemia. J Immunol. 1979 Mar;122(3):1087–1090. [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Breard J. M., Chess L. Triggering of human peripheral blood B cells: polyclonal induction and modulation of an in vitro PFC response. J Immunol. 1976 Nov;117(5 PT2):2021–2028. [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G., Halper J. P., Harris S. R. Induction of in vitro differentiation and immunoglobulin synthesis of human leukemic B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1570–1578. doi: 10.1084/jem.148.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Gmelig-Meyling F., UytdeHaag A. G., Ballieux R. E. Human B-cell activation in vitro. T cell-dependent pokeweed mitogen-induced differentiation of blood B lymphocytes. Cell Immunol. 1977 Sep;33(1):156–169. doi: 10.1016/0008-8749(77)90143-5. [DOI] [PubMed] [Google Scholar]
- Gupta S., Pahwa R., O'Reilly R., Good R. A., Siegal F. P. Ontogeny of lymphocyte subpopulations in human fetal liver. Proc Natl Acad Sci U S A. 1976 Mar;73(3):919–922. doi: 10.1073/pnas.73.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Gomez de la Concha E., Luquetti A., Snajdr M. J., Waxdal M. J., Platts-Mills T. A. T-cell regulation of immunoglobulin synthesis and proliferation in pokeweed (Pa-1)-stimulated human lymphocyte cultures. Scand J Immunol. 1977;6(1-2):109–123. doi: 10.1111/j.1365-3083.1977.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Kay N. E. Abnormal T-cell subpopulation function in CLL: excessive suppressor (T gamma) and deficient helper (T mu) activity with respect to B-cell proliferation. Blood. 1981 Mar;57(3):418–420. [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Levitt D., Duber-Stull D., Lawton A. R. T-cell-dependent and independent plasma cell differentiation induced by Escherichia coli Lipopolysaccharide in human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1981 Mar;18(3):309–321. doi: 10.1016/0090-1229(81)90124-0. [DOI] [PubMed] [Google Scholar]
- Nowell P., Shankey T. V., Finan J., Guerry D., Besa E. Proliferation, differentiation, and cytogenetics of chronic leukemic B lymphocytes cultured with mitomycin-treated normal cells. Blood. 1981 Mar;57(3):444–451. [PubMed] [Google Scholar]
- Pryjma J., Muñoz J., Galbraith R. M., Fudenberg H. H., Virella G. Induction and suppression of immunoglobulin synthesis in cultures of human lymphocytes: effects of pokeweed mitogen and Staphylococcus aureus Cowan I. J Immunol. 1980 Feb;124(2):656–661. [PubMed] [Google Scholar]
- Robert K. H. PHA-induced soluble factor(s) can activate B-cells from patients with chronic lymphatic leukaemia. Clin Exp Immunol. 1979 Sep;37(3):517–522. [PMC free article] [PubMed] [Google Scholar]
- Robèrt K. H., Bird A. G., Möller E. Mitogen-induced differentiation of human CLL lymphocytes to antibody-secreting cells. Scand J Immunol. 1979;10(5):447–452. doi: 10.1111/j.1365-3083.1979.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Del Prete G. F., Maggi E., Falagiani P., Ricci M. T-cell independence of immunoglobulin synthesis by human peripheral blood lymphocytes stimulated with SpA-containing staphylococci. Immunology. 1980 Dec;41(4):921–927. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi G. M., Almerigogna F., Biagiotti R., Bellesi G., Bernardi F., Ricci M. Protein A reactivity of IgM- and IgD-bearing lymphocytes from some patients with chronic lymphocytic leukemia. Clin Immunol Immunopathol. 1981 Apr;19(1):139–148. doi: 10.1016/0090-1229(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Almerigogna F., Ricci M. Interaction of staphylococcal protein A with membrane components of IgM- and/or IgD-bearing lymphocytes from human tonsil. J Immunol. 1980 Apr;124(4):1620–1626. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Saiki O., Ralph P. Induction of human immunoglobulin secretion. I. Synergistic effect of B cell mitogen Cowan I plus T cell mitogens or factors. J Immunol. 1981 Sep;127(3):1044–1047. [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Sirianni M. C., Pandolfi F., Aiuti F., Wigzell H. Protein A-positive staphylococci serve as a selective B cell mitogen for lymphocytes from primary immunodeficiency patients. Clin Exp Immunol. 1979 Apr;36(1):107–111. [PMC free article] [PubMed] [Google Scholar]


