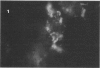
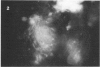
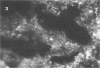
Abstract
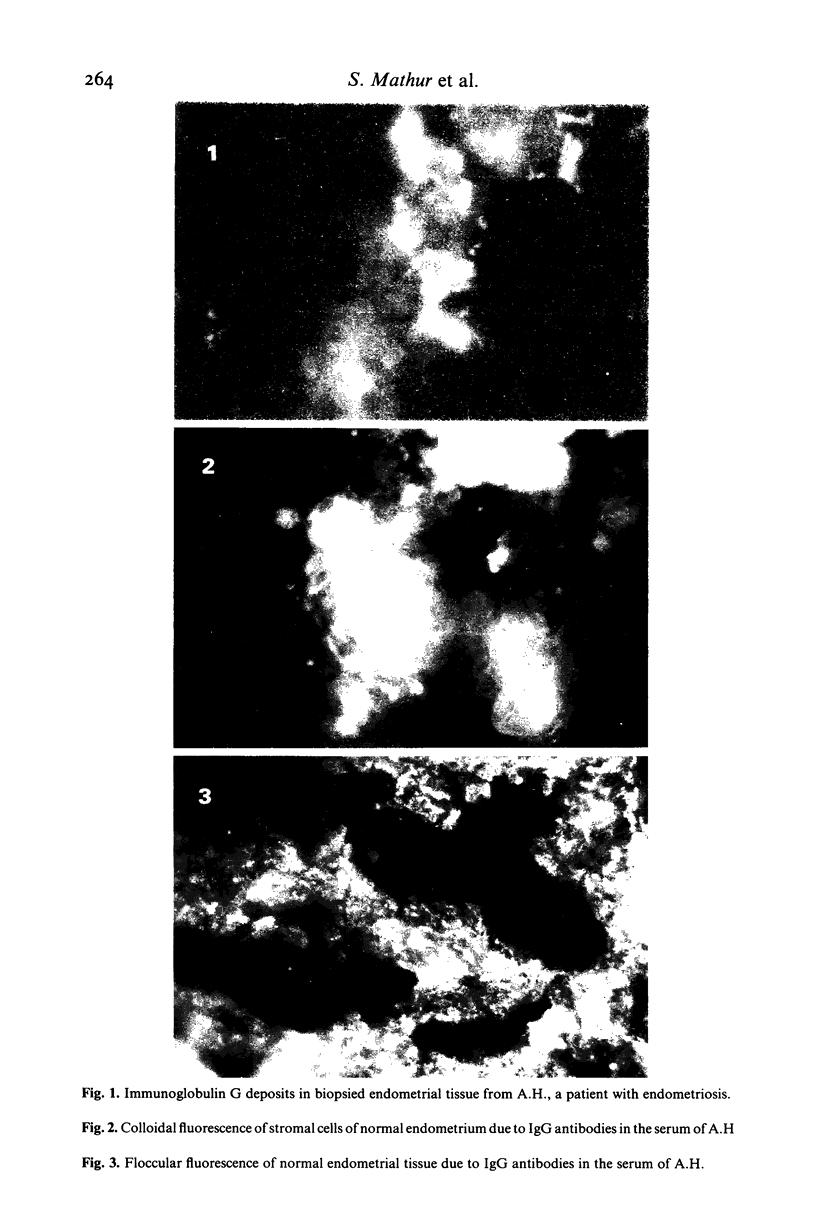
Antibody titres to whole ovary, theca cells, granulosa cells and endometrium were determined by passive haemagglutination and immunofluorescence assays in sera and in cervical and vaginal secretions from 13 patients with endometriosis. Antibody titres to endometrium (mean log2 +/- s.e.m., 7.08 +/- 0.80; P less than 0.0001), ovary (3.58 +/- 0.87; P = 0.0092), theca cells (4.42 +/- 0.73; P less than 0.0001) and granulosa cells (3.33 +/- 0.63; P = 0.0024) were significantly higher in the patients' sera than in sera from 15 normal non-pregnant females. Antibody titres to granulosa cells were elevated (7.97 +/- 1.46; P = 0.0424) in their cervical secretions. Antibody titres to all tissues tested were similar in vaginal secretions of patients and controls. Immunofluorescent antibody assay of biopsied endometrial tissue and sera from the patients revealed the antibodies to be primarily IgG and IgA. The results suggest that autoantibodies to endometrium and ovary are present in patients with endometriosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Channing C. P., Coudert S. P. Contribution of granulosa cells and follicular fluid to ovarian estrogen secretion in rhesus monkey in vivo. Endocrinology. 1976 Mar;98(3):590–597. doi: 10.1210/endo-98-3-590. [DOI] [PubMed] [Google Scholar]
- Coulam C. B., Ryan R. J. Premature menopause. I. Etiology. Am J Obstet Gynecol. 1979 Mar 15;133(6):639–643. doi: 10.1016/0002-9378(79)90011-5. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Autoimmunity and chromosomal aberrations. Am J Hum Genet. 1966 Jan;18(1):93–108. [PMC free article] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Grant A. Additional sterility factors in endometriosis. Fertil Steril. 1966 Jul-Aug;17(4):514–519. doi: 10.1016/s0015-0282(16)36009-5. [DOI] [PubMed] [Google Scholar]
- Kistner R. W., Siegler A. M., Behrman S. J. Suggested classification for endometriosis: relationship to infertility. Fertil Steril. 1977 Sep;28(9):1008–1010. [PubMed] [Google Scholar]
- Lauchlan S. C. The secondary Müllerian system. Obstet Gynecol Surv. 1972 Mar;27(3):133–146. doi: 10.1097/00006254-197203000-00001. [DOI] [PubMed] [Google Scholar]
- Mathur S., Goust J. M., Williamson H. O., Fudenberg H. H. Antigenic cross-reactivity of sperm and T lymphocytes. Fertil Steril. 1980 Nov;34(5):469–476. [PubMed] [Google Scholar]
- Mathur S., Goust J. M., Williamson H. O., Fudenberg H. H. Cross-reactivity of sperm and T lymphocyte antigens. Am J Reprod Immunol. 1981;1(3):113–118. doi: 10.1111/j.1600-0897.1981.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Mathur S., Melchers J. T., 3rd, Ades E. W., Williamson H. O., Fudenberg H. H. Anti-ovarian and anti-lymphocyte antibodies in patients with chronic vaginal candidiasis. J Reprod Immunol. 1980 Dec;2(5):247–262. doi: 10.1016/0165-0378(80)90038-8. [DOI] [PubMed] [Google Scholar]
- Mathur S., Williamson H. O., Derrick F. C., Madyastha P. R., Melchers J. T., 3rd, Holtz G. L., Baker E. R., Smith C. L., Fudenberg H. H. A new microassay for spermocytotoxic antibody: comparison with passive hemagglutination assay for antisperm antibodies in couples with unexplained fertility. J Immunol. 1981 Mar;126(3):905–909. [PubMed] [Google Scholar]
- McNatty K. P., Makris A., DeGrazia C., Osathanondh R., Ryan K. J. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J Clin Endocrinol Metab. 1979 Nov;49(5):687–699. doi: 10.1210/jcem-49-5-687. [DOI] [PubMed] [Google Scholar]
- McNatty K. P., Smith D. M., Makris A., Osathanondh R., Ryan K. J. The microenvironment of the human antral follicle: interrelationships among the steroid levels in antral fluid, the population of granulosa cells, and the status of the oocyte in vivo and in vitro. J Clin Endocrinol Metab. 1979 Dec;49(6):851–860. doi: 10.1210/jcem-49-6-851. [DOI] [PubMed] [Google Scholar]
- Ranney B. Etiology, prevention, and inhibition of endometriosis. Clin Obstet Gynecol. 1980 Sep;23(3):875–882. [PubMed] [Google Scholar]
- Sotsiou F., Bottazzo G. F., Doniach D. Immunofluorescence studies on autoantibodies to steroid-producing cells, and to germline cells in endocrine disease and infertility. Clin Exp Immunol. 1980 Jan;39(1):97–111. [PMC free article] [PubMed] [Google Scholar]
- Soules M. R., Makinak L. R., Bury R., Poindexter A. Endometriosis and anovulation: a coexisting problem in the infertile female. Am J Obstet Gynecol. 1976 Jun 1;125(3):412–417. doi: 10.1016/0002-9378(76)90578-0. [DOI] [PubMed] [Google Scholar]
- Startseva N. V. Kliniko-immunologicheskie aspekty genital'nogo éndometrioza. Akush Ginekol (Mosk) 1980 Mar;(3):23–26. [PubMed] [Google Scholar]
- Weed J. C., Arquembourg P. C. Endometriosis: can it produce an autoimmune response resulting in infertility? Clin Obstet Gynecol. 1980 Sep;23(3):885–893. [PubMed] [Google Scholar]