Abstract
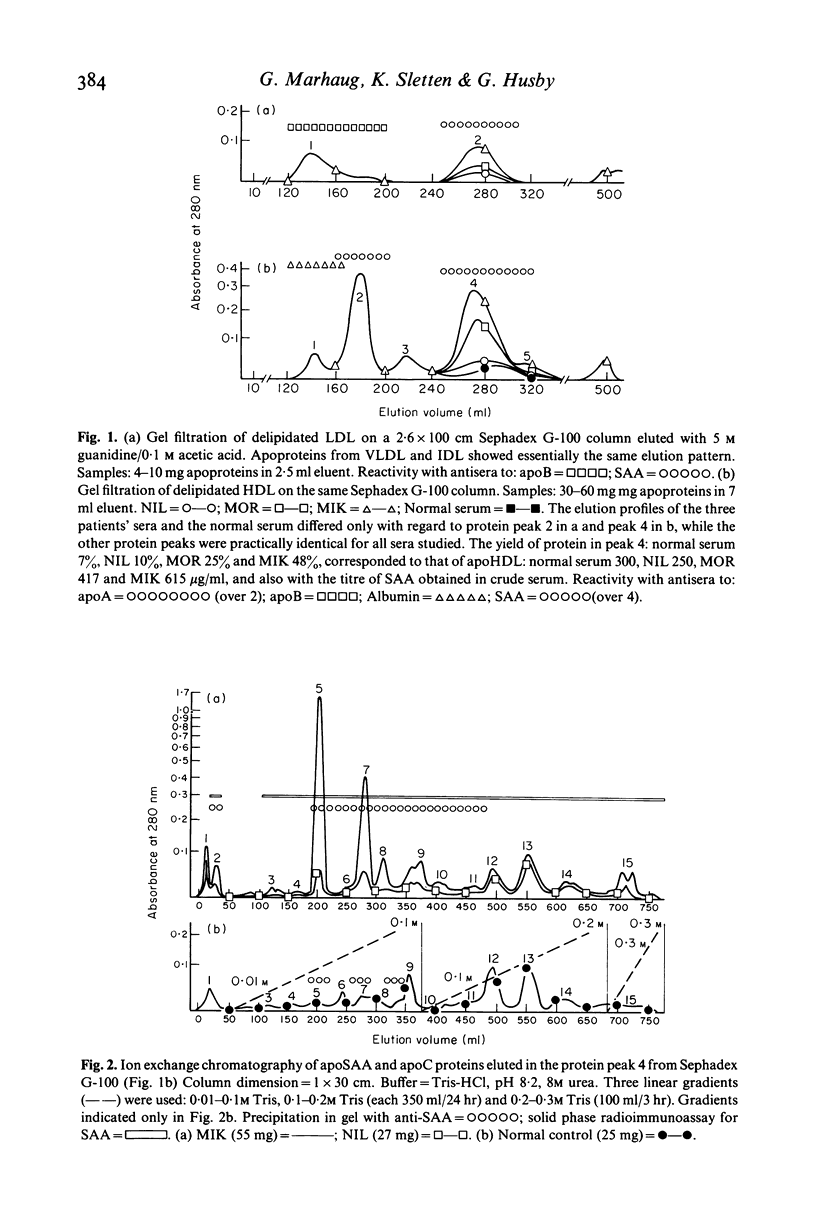
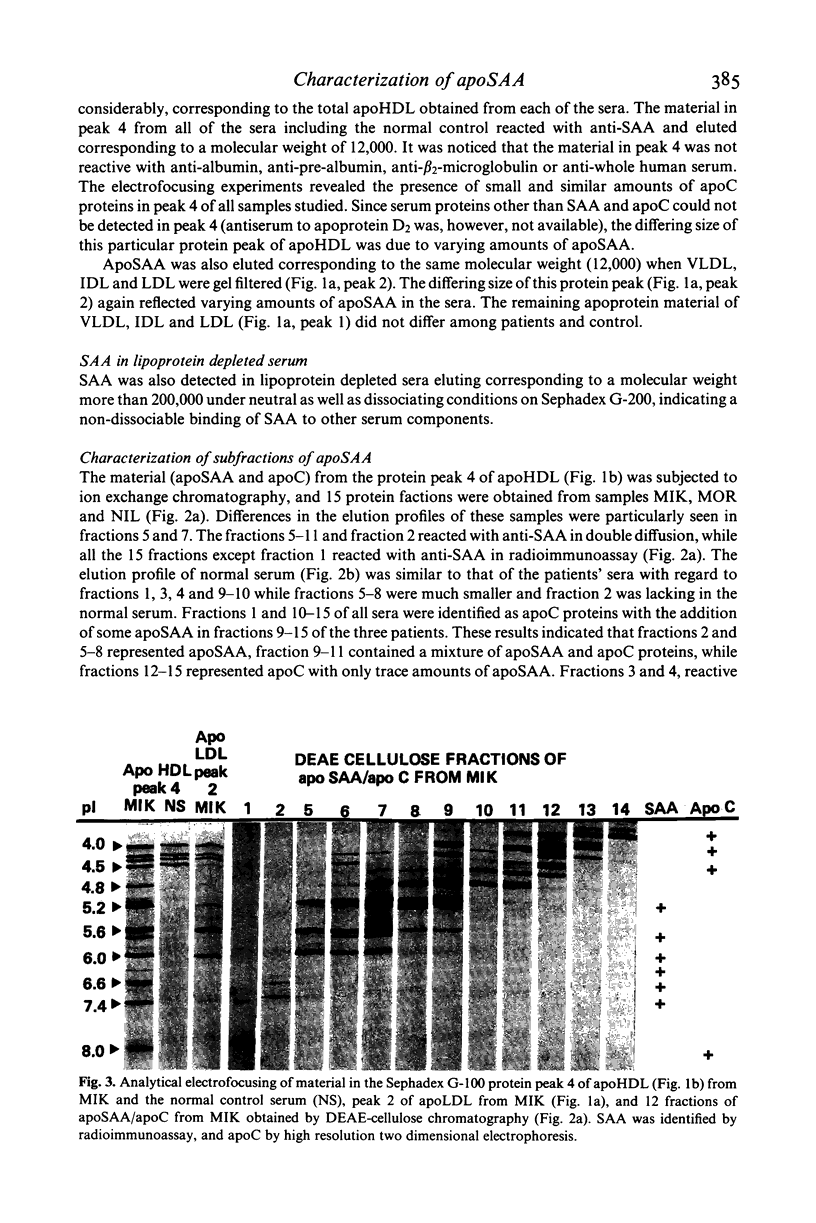
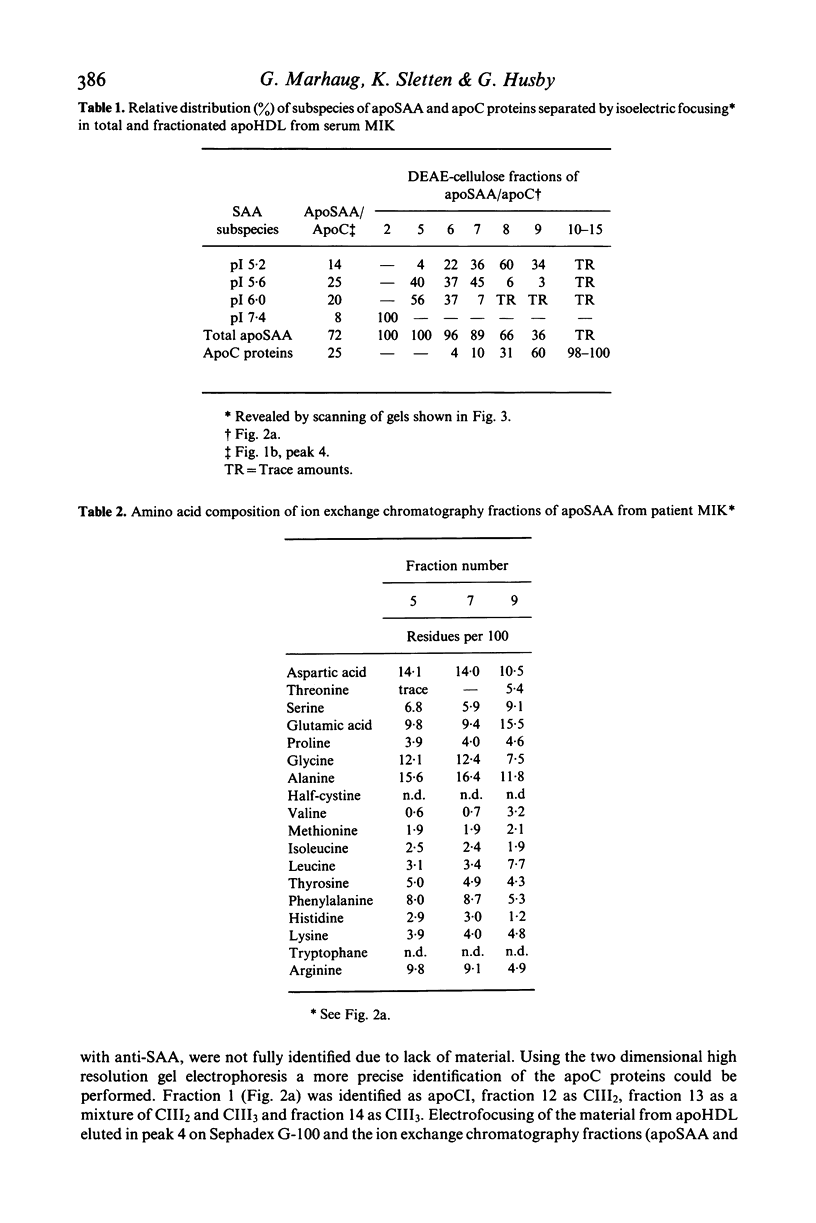
The amyloid related protein SAA was isolated from the serum lipoproteins of three patients with connective tissue disease, one of them having amyloidosis, and from a pool of normal control sera. The bulk of SAA (apoSAA) was complexed to high density lipoprotein (HDL), but significant amounts of apoSAA were also detected in the other lipoprotein fractions. Electrofocusing revealed five-six subspecies of SAA which were distributed in similar proportions in HDL and LDL. Three of these SAA subspecies made up almost all of the apoSAA present in HDL and LDL in the sera from the patients as well as in the control. A small portion of SAA not complexed to lipoproteins was isolated corresponding to a molecular weight higher than 200,000. No particular 'amyloid prone' SAA was found in serum from the patient with amyloidosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Natvig J. B., Michaelsen T. E., Husby G. Isolation and characterization of amyloid-related serum protein SAA as a low molecular weight protein. Scand J Immunol. 1975;4(4):397–401. doi: 10.1111/j.1365-3083.1975.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børresen A. L., Berg K. The apoE polymorphism studied by two-dimensional, high-resolution gel electrophoresis of serum. Clin Genet. 1981 Dec;20(6):438–448. doi: 10.1111/j.1399-0004.1981.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6860–6864. doi: 10.1073/pnas.77.11.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. A serum component related to nonimmunoglobulin amyloid protein AS, a possible precursor of the fibrils. J Clin Invest. 1974 Apr;53(4):1054–1061. doi: 10.1172/JCI107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmendier C. L., Christophe J., Ameryckx J. P. Separation and partial characterization of new apoproteins from human plasma high density lipoproteins. Clin Chim Acta. 1979 Dec 3;99(2):167–176. doi: 10.1016/0009-8981(79)90040-8. [DOI] [PubMed] [Google Scholar]
- Marhaug G., Husby G. Characterization of human amyloid-related protein SAA as a polymorphic protein: association with albumin and prealbumin in serum. Clin Exp Immunol. 1981 Jul;45(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipid-protein interactions in the plasma lipoproteins. Biochim Biophys Acta. 1977 Aug 9;472(2):93–133. doi: 10.1016/0304-4157(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B., Lewis S. B. Isolation and characterization of two threonine-poor apolipoproteins of human plasma high density lipoproteins. Biochemistry. 1978 May 30;17(11):2174–2179. doi: 10.1021/bi00604a023. [DOI] [PubMed] [Google Scholar]
- Skogen B., Børresen A. L., Natvig J. B., Berg K., Michaelsen T. E. High-density lipoprotein as carrier for amyloid-related protein SAA in rabbit serum. Scand J Immunol. 1979;10(1):39–45. doi: 10.1111/j.1365-3083.1979.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Sletten K., Natvig J. B., Husby G., Juul J. The complete amino acid sequence of a prototype immunoglobulin-lambda light-chain-type amyloid-fibril protein AR. Biochem J. 1981 Jun 1;195(3):561–572. doi: 10.1042/bj1950561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]




