Abstract
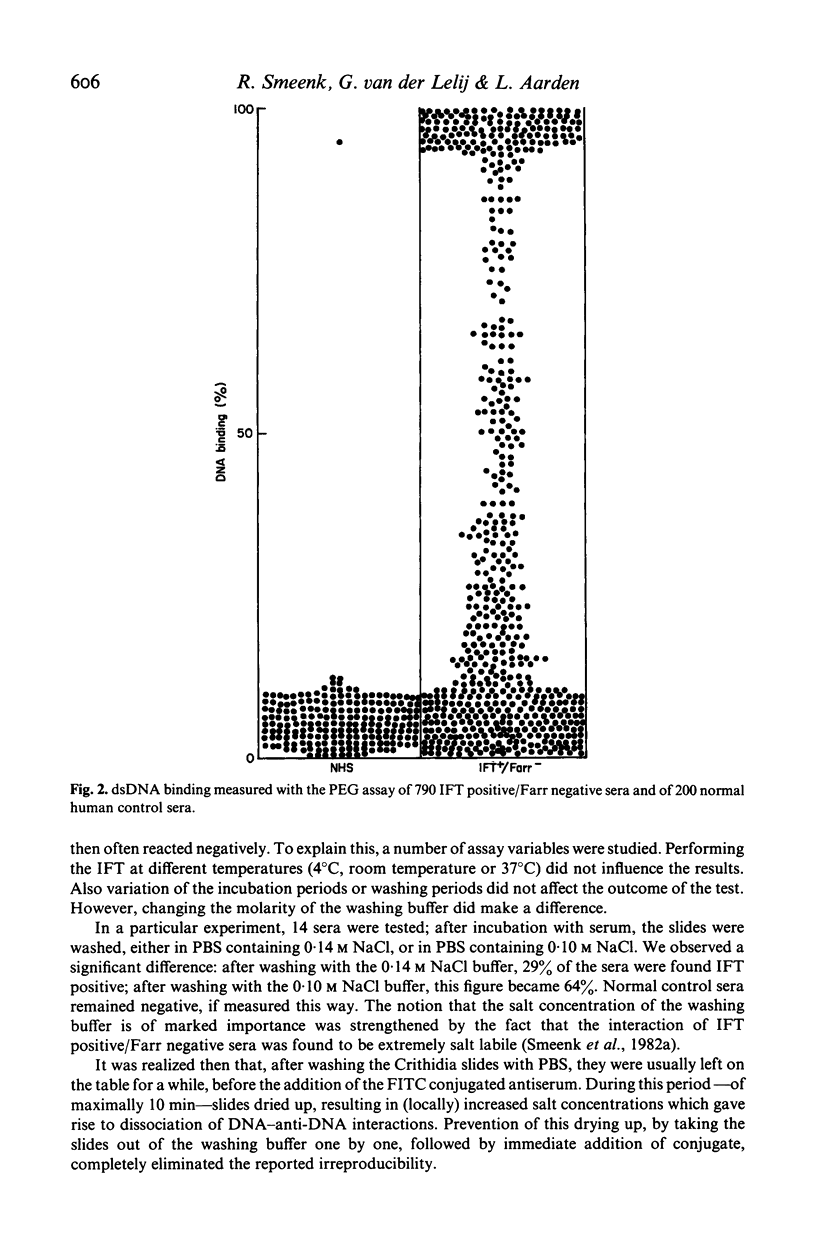
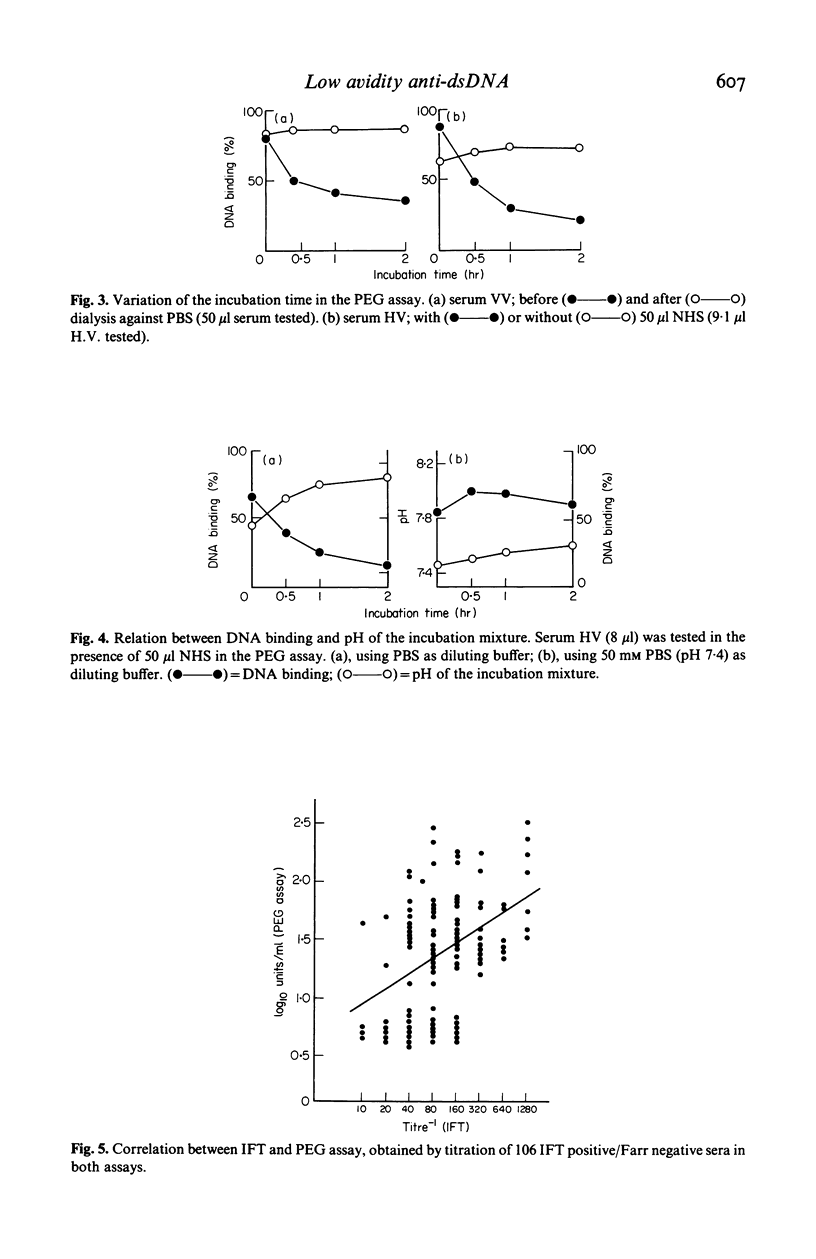
With the immunofluorescence technique (IFT) using Crithidia luciliae as a substrate, 14,417 sera sent to our laboratory for routine anti-dsDNA determination, were screened for the presence of antibodies to dsDNA. The 1,260 sera that were found IFT positive were then assayed with the Farr radioimmunoassay, in which 3H-labelled PM2-DNA is used as antigen. Only 470 sera (37%) were found to be Farr positive. This discrepancy is, at least partially, caused by the fact that the Farr assay does not detect anti-DNA of low avidity, whereas the Crithidia-IFT does. Sixty-eight percent of the IFT-positive/Farr negative sera were found positive with the PEG assay, a radioimmunoassay that also employs double stranded PM2-DNA as antigen, and that also detects anti-dsDNA of low avidity. The IFT performed on IFT positive/Farr negative sera was found to be rather irreproducible. It was shown that this was due to local increases of the salt concentration resulting from the way the assay was performed. The problem could be overcome by careful control of the assay conditions, i.e. never letting Crithidia slides dry up after washing with PBS. In the PEG assay, these sera sometimes showed a DNA binding that decreased with time. It could be shown that this is caused by a parallel increase in pH during the incubation as a result of CO2 evaporation from the serum.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., Lakmaker F., De Groot E. R. Immunology of DNA. IV. Quantitative aspects of the Farr assay. J Immunol Methods. 1976;11(2):153–163. doi: 10.1016/0022-1759(76)90143-5. [DOI] [PubMed] [Google Scholar]
- Aarden L. A., Lakmaker F., Feltkamp T. E. Immunology of DNA. I. The influence of reaction conditions on the Farr assay as used for the detection of anti-ds DNA. J Immunol Methods. 1976;10(1):27–37. doi: 10.1016/0022-1759(76)90004-1. [DOI] [PubMed] [Google Scholar]
- Aarden L. A., de Groot E. R., Feltkamp T. E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975 Jun 30;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- Ballou S. P., Kushner I. Immunochemical characteristics of antibodies to DNA in patients with active systemic lupus erythematosus. Clin Exp Immunol. 1979 Jul;37(1):58–67. [PMC free article] [PubMed] [Google Scholar]
- Bruneau C., Benveniste J. Circulating DNA:anti-DNA complexes in systemic lupus erythematosus. Detection and characterization by ultracentrifugation. J Clin Invest. 1979 Jul;64(1):191–198. doi: 10.1172/JCI109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe W., Kushner I. An immunofluorescent method using Crithidia luciliae to detect antibodies to double-stranded DNA. Arthritis Rheum. 1977 Apr;20(3):811–814. doi: 10.1002/art.1780200308. [DOI] [PubMed] [Google Scholar]
- Deegan M. J. Systemic lupus erythematosus. Some contemporary laboratory aspects. Arch Pathol Lab Med. 1980 Aug;104(8):399–404. [PubMed] [Google Scholar]
- Ebling F., Hahn B. H. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980 Apr;23(4):392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Steinberg A. D. Qualitative characteristics of anti-DNA antibodies in lupus nephritis. Arthritis Rheum. 1974 Nov-Dec;17(6):947–954. doi: 10.1002/art.1780170605. [DOI] [PubMed] [Google Scholar]
- Griffiths I. D., Dick W. C. Antibodies to DNA antigens: their specificity and clinical relevance. Eur J Clin Invest. 1979 Aug;9(4):239–241. doi: 10.1111/j.1365-2362.1979.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Koffler D., Agnello V., Thoburn R., Kunkel H. G. Systemic lupus erythematosus: prototype of immune complex nephritis in man. J Exp Med. 1971 Sep 1;134(3 Pt 2):169s–179s. [PubMed] [Google Scholar]
- Leon S. A., Green A., Ehrlich G. E., Poland M., Shapiro B. Avidity of antibodies in SLE: relation to severity of renal involvement. Arthritis Rheum. 1977 Jan-Feb;20(1):23–29. doi: 10.1002/art.1780200104. [DOI] [PubMed] [Google Scholar]
- Monier J. C., Perraud M., Picard F., Gioud M. Comparison of three techniques for the detection of antibodies to double-stranded DNA: immunofluorescence on Trypanosoma gambiense, immunofluorescence on Crithidia luciliae and radioimmunoassay using the Farr technique. Ann Immunol (Paris) 1978 Apr-Jun;129 100(4):463–474. [PubMed] [Google Scholar]
- Riley R. L., Addis D. J., Taylor R. P. Stability of DNA/anti-DNA complexes. II. Salt lability and avidity. J Immunol. 1980 Jan;124(1):1–7. [PubMed] [Google Scholar]
- Riley R. L., McGrath H., Jr, Taylor R. P. Detection of low avidity anti-DNA antibodies in systemic lupus erythematosus. Arthritis Rheum. 1979 Mar;22(3):219–225. doi: 10.1002/art.1780220303. [DOI] [PubMed] [Google Scholar]
- Slater N. G., Cameron J. S., Lessof M. H. The Crithidia luciliae kinetoplast immunofluorescence test in systemic lupus erythematosus. Clin Exp Immunol. 1976 Sep;25(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Smeenk R., Aarden L. The use of polyethylene glycol precipitation to detect low-avidity anti-DNA antibodies in systemic lupus erythematosus. J Immunol Methods. 1980;39(1-2):165–180. doi: 10.1016/0022-1759(80)90305-1. [DOI] [PubMed] [Google Scholar]
- Smeenk R., van der Lelij G., Aarden L. Avidity of antibodies to dsDNA: comparison of IFT on Crithidia luciliae, Farr assay, and PEG assay. J Immunol. 1982 Jan;128(1):73–78. [PubMed] [Google Scholar]
- Sontheimer R. D., Gilliam J. N. An immunofluorescence assay for double-stranded DNA antibodies using the Crithidia luciliae kinetoplast as a double-stranded DNA substrate. J Lab Clin Med. 1978 Apr;91(4):550–558. [PubMed] [Google Scholar]
- Stingl G., Meingassner J. G., Swelty P., Knapp W. An immunofluorescence procedure for the demonstration of antibodies to native, double-stranded DNA and of circulating DNA-anti-DNA complexes. Clin Immunol Immunopathol. 1976 Sep;6(2):131–140. doi: 10.1016/0090-1229(76)90103-3. [DOI] [PubMed] [Google Scholar]
- Swaak A. J., Aarden L. A., Statius van Eps L. W., Feltkamp T. E. Anti-dsDNA and complement profiles as prognostic guides in systemic lupus erythematosus. Arthritis Rheum. 1979 Mar;22(3):226–235. doi: 10.1002/art.1780220304. [DOI] [PubMed] [Google Scholar]
- Swaak A. J., Groenwold J., Aarden L. A., Feltkamp T. E. Detection of anti-dsDNA as diagnostic tool. Ann Rheum Dis. 1981 Feb;40(1):45–49. doi: 10.1136/ard.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold R. T., Young F. E., Tan E. M., Farr R. S. Deoxyribonucleic acid antibody: a method to detect its primary interaction with deoxyribonucleic acid. Science. 1968 Aug 23;161(3843):806–807. doi: 10.1126/science.161.3843.806. [DOI] [PubMed] [Google Scholar]
- de Groot E. R., Lamers M. C., Aarden L. A., Smeenk R. J., van Oss C. J. Dissociation of DNA/anti-DNA complexes at high pH. Immunol Commun. 1980;9(5):515–528. doi: 10.3109/08820138009066012. [DOI] [PubMed] [Google Scholar]


