Abstract
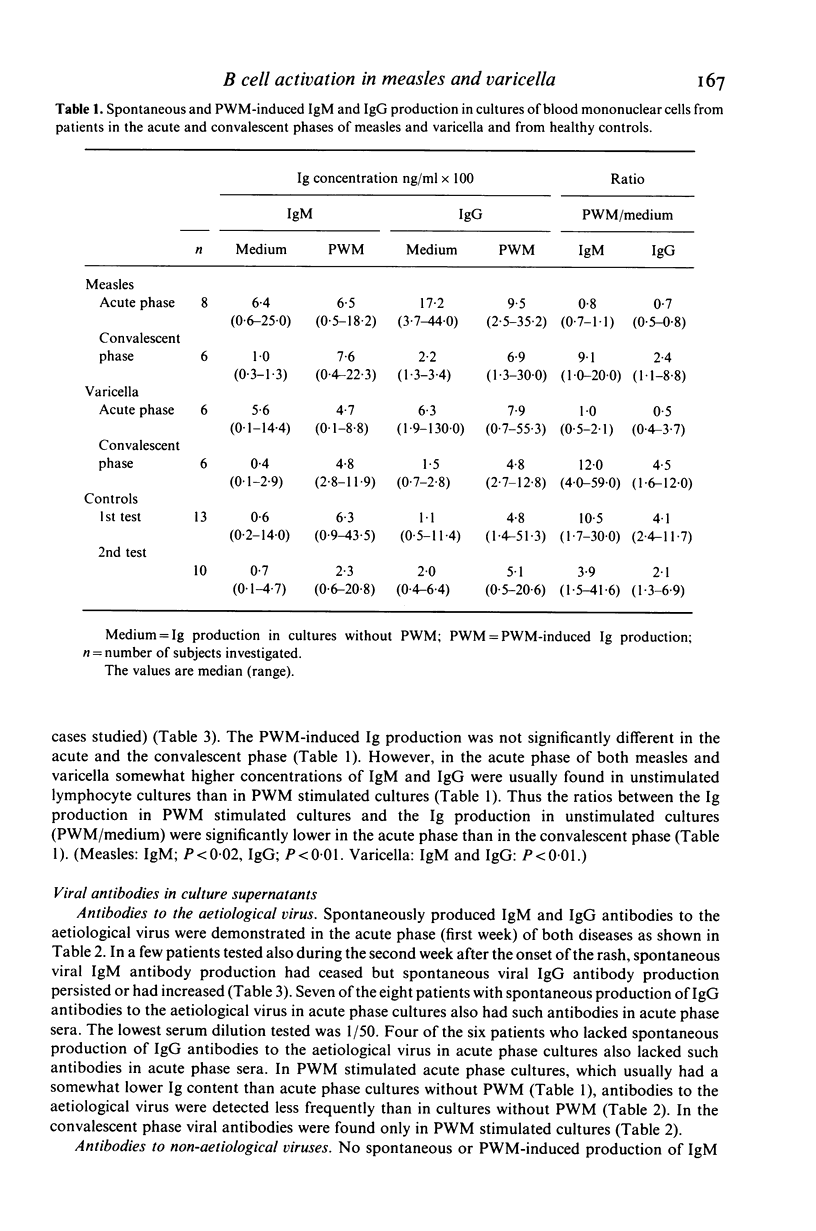
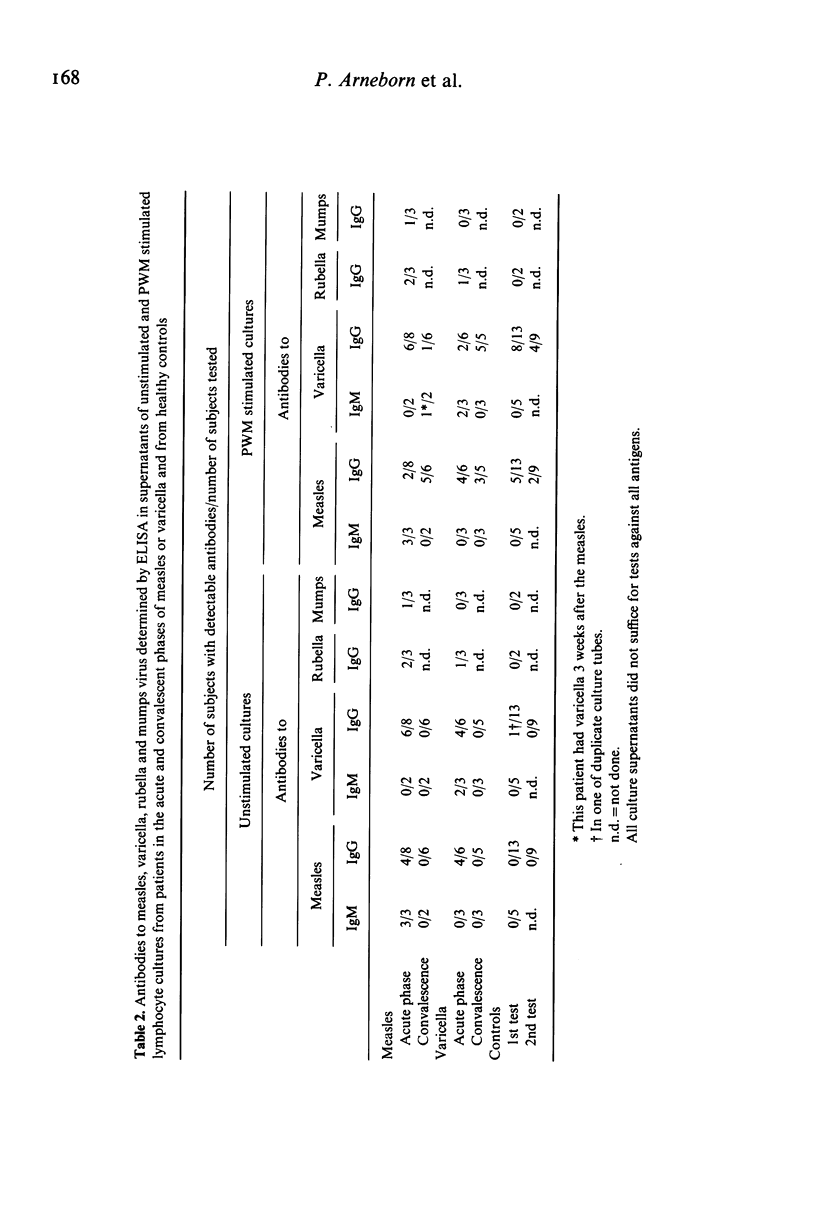
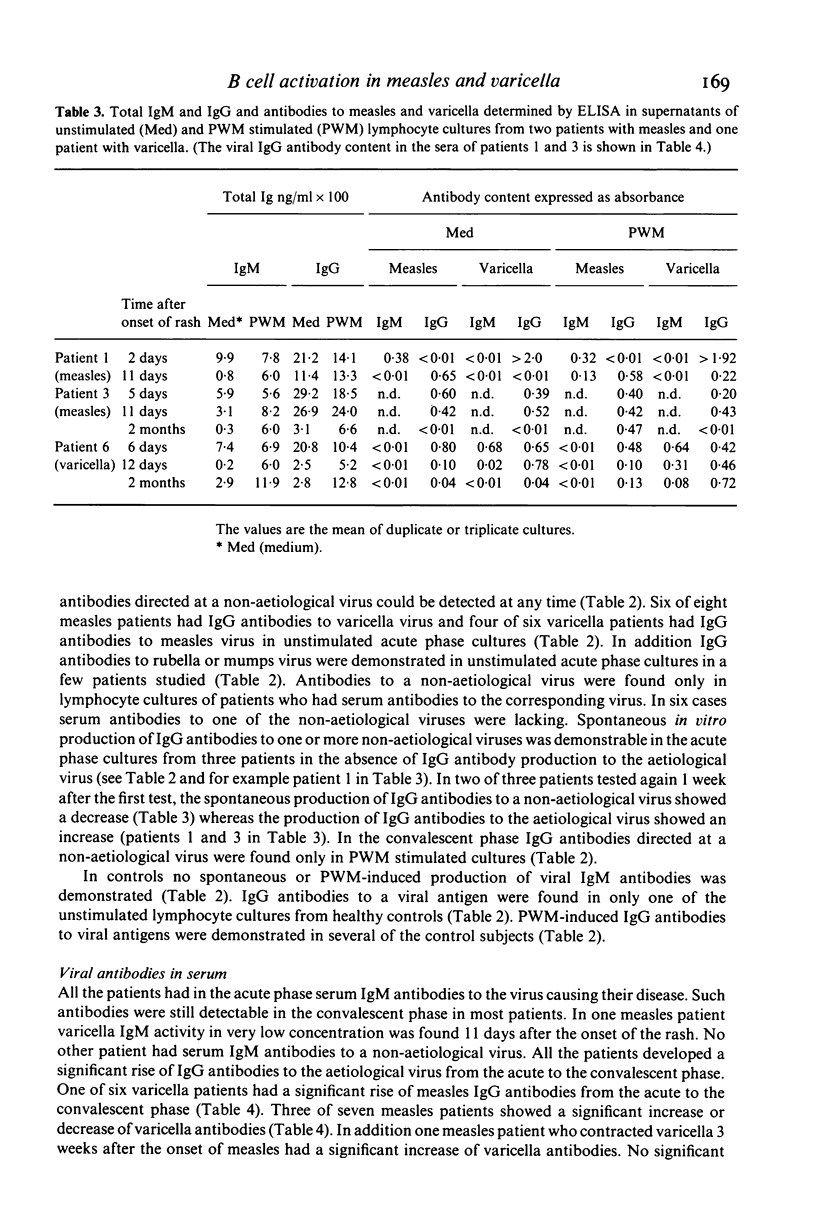
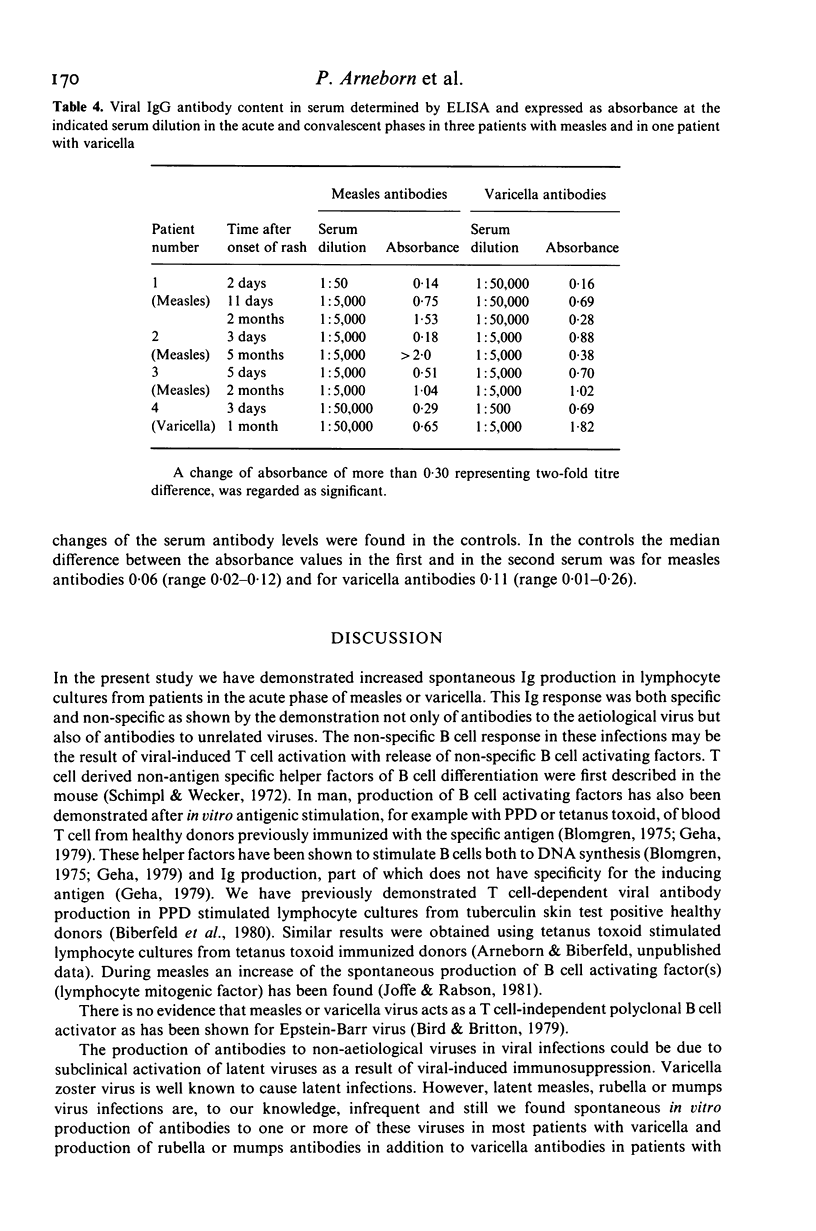
Lymphocytes from eight patients with measles and six patients with varicella were studied during the acute phase (first week) of illness and after recovery for spontaneous and pokeweed mitogen (PWM)-induced production of immunoglobulins (Ig) and viral antibodies by an enzyme linked immunosorbent assay (ELISA). In both infections acute phase lymphocytes showed increased spontaneous in vitro IgM and IgG productions including IgM and IgG antibodies to the aetiological virus as well as IgG antibodies to unrelated viruses (varicella, measles, rubella and mumps) to which the patient had serum antibodies. PWM induced no further Ig synthesis in the acute phase. In the convalescent phase viral antibody production could be demonstrated only in PWM stimulated cultures. In four patients the spontaneous synthesis of antibodies to a non-aetiological virus seemed to precede the production of IgG antibodies to the aetiological virus. All patients showed an increase of ELISA determined serum antibodies to the aetiological virus from the acute to the convalescent phase. Three of seven measles patients also showed a minor but significant increase or decrease of serum IgG antibodies to varicella and one of six varicella patients a significant rise of serum IgG antibodies to measles. Thus both measles and varicella infections were associated with non-specific as well as specific B cell activation. The non-specific B cell activation may be induced by non-specific helper factors from activated T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arneborn P., Biberfeld G., von Stedingk L. V. T lymphocyte subpopulations defined by monoclonal antibodies and FC receptor binding in relation to immunosuppression in vaccine-induced rubella infection. Acta Pathol Microbiol Immunol Scand C. 1982 Jun;90(3):163–170. doi: 10.1111/j.1699-0463.1982.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Biberfeld G., Forsgren M., Von Stedingk L. V., Arneborn P. PPD-induced viral antibody production in human blood lymphocytes. Clin Exp Immunol. 1980 Nov;42(2):364–369. [PMC free article] [PubMed] [Google Scholar]
- Bird A. G., Britton S. A live human B-cell activator operating in isolation of other cellular influences. Scand J Immunol. 1979;9(6):507–510. doi: 10.1111/j.1365-3083.1979.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Blomgren H. Role of B cells in the expression of the PPD response of human lymphocytes in vitro. Scand J Immunol. 1975 Sep;4(5-6):499–510. doi: 10.1111/j.1365-3083.1975.tb02655.x. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Dennis J. Antibody assays for varicella-zoster virus: comparison of enzyme immunoassay with neutralization, immune adherence hemagglutination, and complement fixation. J Clin Microbiol. 1978 Nov;8(5):545–552. doi: 10.1128/jcm.8.5.545-552.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S. Regulation of human B cell activation. Immunol Rev. 1979;45:275–305. doi: 10.1111/j.1600-065x.1979.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Defective helper factor (LMF) production in patients with acute measles infection. Clin Immunol Immunopathol. 1981 Aug;20(2):215–223. doi: 10.1016/0090-1229(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Moticka E. J., Streilein J. W. Hypothesis: nonspecific polyclonal activation of memory B cells by antigen as a mechanism for the preservation of long term immunologic anamnesis. Cell Immunol. 1978 Dec;41(2):406–413. doi: 10.1016/0008-8749(78)90237-x. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Vandvik B., Nilsen R. E., Vartdal F., Norrby E. Mumps meningitis: specific and non-specific antibody responses in the central nervous system. Acta Neurol Scand. 1982 May;65(5):468–487. doi: 10.1111/j.1600-0404.1982.tb03104.x. [DOI] [PubMed] [Google Scholar]
- Wasserman J., Von Stedingk L. V., Biberfeld G., Petrini B., Blomgren H., Baral E. The effect of irradiation on T-cell suppression of ELISA-determined Ig production by human blood B-cells in vitro. Clin Exp Immunol. 1979 Nov;38(2):366–369. [PMC free article] [PubMed] [Google Scholar]


