Abstract
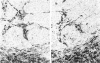
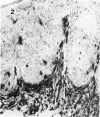
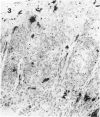
Monoclonal antibodies, directed against functionally different lymphocyte subsets, were applied on frozen sections of primary malignant melanomas and benign nevi. Positive reaction was identified by means of an immunoperoxidase method. It was found that lymphocytic infiltrate underneath and in between malignant melanoma is composed of approximately equal numbers of OKT4 positive helper and OKT8 positive suppressor/cytotoxic T cells. The majority of these lymphocytes also expressed HLA-Dr antigen, indicating an activated state. In addition HLA-Dr, OKT6 positive dendritic cells were present in the infiltrate and between the melanoma cells. Finally, melanoma cells expressed demonstrable amounts of HLA A, B, C antigens, whereas benign nevi did not. It is concluded that all ingredients for a successful immune reaction against primary malignant melanoma are on hand. This finding is in agreement with the relatively frequent occurrence of partial or even complete regression of primary malignant melanoma of the skin.
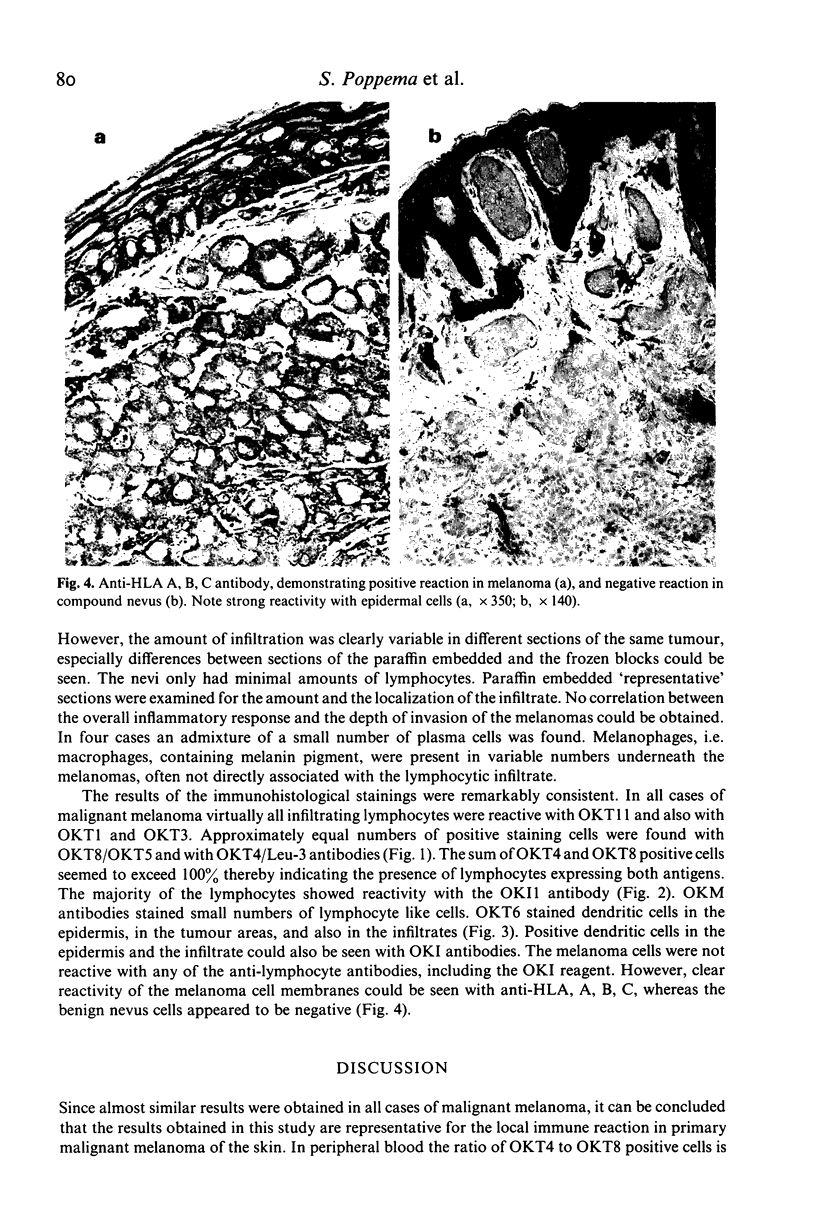
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Dienstag J. L., Wands J. R., Schlossman S. F., Reinherz E. L. Alterations of T-cell subsets in primary biliary cirrhosis. Clin Exp Immunol. 1982 Feb;47(2):351–358. [PMC free article] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Cosimi A. B., Colvin R. B., Burton R. C., Rubin R. H., Goldstein G., Kung P. C., Hansen W. P., Delmonico F. L., Russell P. S. Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med. 1981 Aug 6;305(6):308–314. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Mihm M. C., Jr, Osage J. E., Dvorak H. F. Melanoma. An ultrastructural study of the host inflammatory and vascular responses. J Invest Dermatol. 1980 Nov;75(5):388–393. doi: 10.1111/1523-1747.ep12523627. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Howe A. J., Seeger R. C., Molinaro G. A., Ferrone S. Analysis of human tumor cells for Ia-like antigens with monoclonal antibodies. J Natl Cancer Inst. 1981 May;66(5):827–829. [PubMed] [Google Scholar]
- Kaszubowski P. A., Husby G., Tung K. S., Williams R. C., Jr T-lymphocyte subpopulations in peripheral blood and tissues of cancer patients. Cancer Res. 1980 Dec;40(12):4648–4657. [PubMed] [Google Scholar]
- Kay H. D., Horwitz D. A. Evidence by reactivity with hybridoma antibodies for a probable myeloid origin of peripheral blood cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Clin Invest. 1980 Oct;66(4):847–851. doi: 10.1172/JCI109923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern V. J. Spontaneous regression of melanoma. Pathology. 1975 Apr;7(2):91–99. doi: 10.3109/00313027509092702. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Ting A., Zweerink H. J., Askonas B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature. 1977 Dec 8;270(5637):524–526. doi: 10.1038/270524a0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. Surface markers of cloned human T cells with various cytolytic activities. J Exp Med. 1981 Aug 1;154(2):569–574. doi: 10.1084/jem.154.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Pesando J. M., Ritz J., Goldstein G., Schlossman S. F. Ia determinants on human T-cell subsets defined by monoclonal antibody. Activation stimuli required for expression. J Exp Med. 1979 Dec 1;150(6):1472–1482. doi: 10.1084/jem.150.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Jr, Stehlin J. S., Jr Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965 Nov;18(11):1399–1415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Szekeres L., Daróczy J. Electron microscopic investigation on the local cellular reaction to primary malignant melanoma. Dermatologica. 1981;163(2):137–144. doi: 10.1159/000250150. [DOI] [PubMed] [Google Scholar]