Abstract
Background
Online information about the availability of clinical trials promises to enhance the accrual of patients into trials. The primary objective of our study was to assess the completeness of online databases of breast cancer clinical trials available in Canada.
Methods
Eligible online resources were identified through a search of MEDLINE (articles published from 1966 to January 2002), an Internet search with Google, examination of Web sites of cancer organizations and information from experts. Resources were included if they contained information about open, active cancer clinical trials available in Canada. Web sites reviewed were not limited to those based in Canada. For each eligible resource, the number of listed trials and the proportion of trials identified were calculated.
Results
Of 30 Web sites identified, 8 met all of the inclusion criteria; 3 were based in Canada and 5 in the United States. The total number of breast cancer trials identified as being available in Canada was 28. The Physician Data Query (PDQ) Clinical Trials Database of the US National Cancer Institute (cancer.gov/search/clinical_trials) identified 86% (24/28). The database of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) (ctg.queensu.ca) identified 29% (8/28) of the available breast cancer trials. CenterWatch Clinical Trials Listing Service (centerwatch.com) identified 4% (1/28).
Interpretation
If the PDQ database included all of the NCIC CTG trials, it would become the most complete database of breast cancer clinical trials currently available in Canada. Online cancer data sources should strive to make access to clinical trials simpler and more reliable, particularly for residents of the country where the trial is to be conducted.
The number of Web sites that provide information about clinical trials has grown rapidly. Many of these sites are designed to enhance the understanding of clinical trials by the public and to provide information about the availability of trials to potential participants, regardless of their location. Cancer is an area that is ideally suited to benefit from this novel approach.
The number of adult patients with common cancers who receive treatment as part of a formal clinical trial in North America is estimated to be quite low (perhaps less than 5% of cancer patients).1,2 There is extensive literature on the factors known to be associated with low accrual into cancer clinical trials.2,3 Of importance to potential participants are limited knowledge of trial availability and of eligibility criteria for specific trials, and the need to travel to interact with the research groups that are coordinating the trials.2,3,4,5
The primary objective of our study was to assess the completeness of online databases of cancer clinical trials, with the example of breast cancer, and to promote awareness of the need for better online databases about clinical trials in general. This can be regarded as a case study on the diffusion of innovations.6,7
Methods
Web sites were included for review if they provided free access to searchable online databases that contained information about individual cancer clinical trials (phase I through phase IV) available in Canada. These sites did not need to be designed or located in Canada. We excluded sites designed or located outside of Canada that did not provide access to information about cancer clinical trials available in Canada.
Eligible Web sites were identified by the following means:
· A search of MEDLINE for articles published from 1966 to January 2002, with the terms “randomized controlled trials,” “clinical trials,” “database,” “cancer,” “oncology” and “Canada” as text words and, whenever possible, as MeSH (medical subheading) terms.
· A search for the first 200 Web sites identified using the search engine Google (www.google.com) with the key words “clinical,” “trial,” “database,” “cancer” and “Canada.” Google was selected because it is the largest search engine available, and it yields results that are ranked by their relevance.
· A search of the Web sites of major organizations that promote or support cancer care, such as the National Cancer Institute of Canada and the US National Cancer Institute, the American Society of Clinical Oncology, the American and Canadian Cancer Societies, the Program in Evidence-based Care of Cancer Care Ontario, OncoLink, the US National Institutes of Health and the Canadian Institutes of Health Research.
· A review of a list of “link” Web sites (ones that provide Internet links to other Web sites about clinical trials), including those already known to the authors or recommended to them by members of the planning group for a new clinical trials information network, to be supported by the Ontario Cancer Research Network (www.ocrn.org).
From the eligible Web sites, 2 of us (J.E.T. and R.A.P.) extracted information independently, using the information available online. The 2 of us each logged onto all of the eligible sites and extracted information about the name, URL and country of origin of the organization responsible for the Web site; the focus of the web site (cancer-specific or general); the number of cancer clinical trials listed as currently in progress (open or active) in Canada; and the number of breast cancer clinical trials listed as currently in progress (open or active) in Canada. Data on the last 2 items were obtained by searching the database of the site with the term “Canada” and, when appropriate, “cancer.” The 2 of us produced a common data set by consensus. Any disagreements were resolved through discussion with the other author (A.R.J.). Closed trials and those still being planned were excluded. All of the data reported in this article were collected on Apr. 5, 2002.
The number of unique trials on any type of cancer, and on breast cancer in particular, identified from all of the databases as being available in Canada was obtained by eliminating duplicate entries. For each of the Web sites that were reviewed, we calculated the proportion of available breast cancer clinical trials relative to the total number of cancer clinical trials available. The total number of breast cancer trials was used as the denominator to calculate the proportion of breast cancer trials identified by each of the Web sites. The ideal denominator would have included privately sponsored and publicly funded trials designed or conducted at all institutions in Canada.
Results
We identified 30 Web sites, of which 8 met all of the inclusion criteria. Three were based in Canada and 5 in the United States. Three yielded unique Canadian breast cancer trials (Table 1).
Table 1
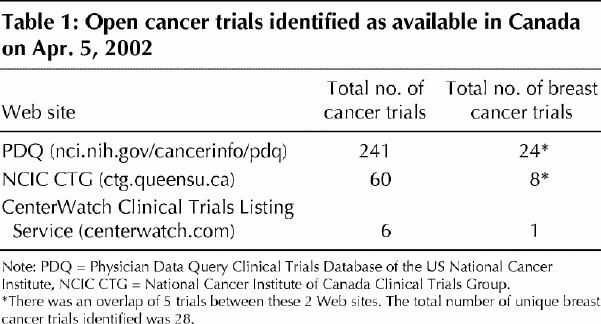
The database of Canadian cancer trials of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) (www.ctg.queensu.ca) was created for clinical trials sponsored by the NCIC and in progress in Canada. Searching it yielded descriptions of 48 phase III and 12 phase I or II active trials (at the time of review in April 2002, the database had been updated as of Nov. 30, 2001). Of the 60 open trials, 8 (13%) were trials of breast cancer; none of the phase I or II trials was specifically of breast cancer.
The Physician Data Query (PDQ) Clinical Trials Database of the US National Cancer Institute (www.cancer.gov/search/clinical_trials) allows one to search by type of cancer and by location of trial (city and country). Searching it for cancer trials (including phase I and II trials) in Canada yielded a total of 241 active trials, of which 24 (10%) were breast cancer trials. The information on all of the trials included in PDQ was also available in ClinicalTrials.gov (www.clinicaltrials.gov), a registry of the US National Institutes of Health (NIH) developed by its National Library of Medicine. ClinicalTrials.gov contains information on federally and privately funded clinical trials of experimental treatments for serious or life-threatening diseases funded by NIH institutes, other federal agencies, the pharmaceutical industry, and academic or other nonprofit organizations.
Searching the CenterWatch.com Web site (www.centerwatch.com/search.asp) using the keyword “cancer” and the trial location “Canada” yielded 6 trials at 45 locations in Canada. One novel breast cancer trial, available in Canada at 28 locations, was identified.
A total of 33 breast cancer trials were identified as being available in Canada (24 in the PDQ database, 8 in the NCIC CTG database and 1 in the CenterWatch.com database). Five of the trials were listed on both the PDQ and NCIC CTG sites, for a total of 28 unique breast cancer trials (24 [86%] on the PDQ site, 8 [29%] on the NCIC CTG site and 1 [4%] on CenterWatch.com).
Other sites were identified that listed breast cancer trials, but none described a unique Canadian trial not already listed in the preceding 3 Web sites. Current Controlled Trials (www.controlled-trials.com/mrct) uses ClinicalTrials .gov as its main source of cancer clinical trials data.8 Cancer411.org (www.cancer411.org/clinicaltrials/index.asp), which obtains part of its information from PDQ, did not yield additional trials. The bettercancercare.com Web site (www.bettercancercare.com) seems to have been developed in Canada, but at the time of our study it was not possible to search for trials available at a particular location. The MediStudy.com Web site (www.medistudy.com/clinical_trials/index.html) is intended to provide access to information about clinical trials in Canada, but at the time of our review no cancer trials were registered.
Interpretation
Our results suggest that there is no comprehensive online database of cancer clinical trials available in Canada. Indeed, the best source we could find of Canadian breast cancer trials was the PDQ database, a US-based initiative. A recent study, however, showed that PDQ is far from comprehensive, since it did not include information about all US-based trials on prostate or colon cancer.9
Our study is limited by its exploratory nature, its focus on breast cancer and the lack of a comprehensive list of Canadian breast cancer clinical trials against which the online databases could have been compared. However, we believe that our data provide a good reflection of the current situation of online databases of clinical trials, at least in the cancer field.
Our findings underscore the need for more efforts to study the role of online databases of clinical trials and to determine whether such databases could help overcome some of the problems that, until now, have prevented the creation of comprehensive databases of clinical trials.10 Future studies should explore different incentives to motivate the online listing of trials, particularly those that are privately sponsored. The US Food and Drug Administration now requires that information be submitted to the ClinicalTrials.gov Web site about all clinical trials under an investigational new drug application, particularly for the evaluation of drugs to treat serious or life-threatening conditions.11 Canada may benefit from a similar approach, if proven enforceable in the United States.
Online databases of clinical trials also create opportunities for further research on the desired characteristics of such Web sites, on their perceived importance by potential participants and their health care providers, an on the impact of new technologies to improve the accessibility of information and the recruitment of participants into clinical trials.12,13,14,15
Footnotes
This article has been peer reviewed.
Contributors: All authors made substantial contributions to the conception and design of the study, and to the process of data acquisition, analysis and interpretation. All of the authors contributed substantially to drafting the article and revising it for important intellectual content and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Alejandro R. Jadad, Centre for Global eHealth Innovation, 4th floor, R. Fraser Elliott Building, Toronto General Hospital, 190 Elizabeth St., Toronto ON M5G 2C4; fax 416 340-3595; ajadad@uhnres.utoronto.ca
References
- 1.Fisher B. On clinical trial participation. J Clin Oncol 1991;9:1927-30. [DOI] [PubMed]
- 2.Ellis PM, Butow PN, Tattersall MH, Dunn SM, Houssami N. Randomized clinical trials in oncology: Understanding and attitudes predict willingness to participate. J Clin Oncol 2001;19:3554-61. [DOI] [PubMed]
- 3.Gotay CC. Accrual to cancer clinical trials: directions from the research literature. Soc Sci Med 1991;33:569-77. [DOI] [PubMed]
- 4.Sutherland HJ, Carlin K, Harper W, Martin LJ, Greenberg CV, Till JE, et al. A study of diet and breast cancer prevention in Canada: why healthy women participate in controlled trials. Cancer Causes Control 1993;4:521-8. [DOI] [PubMed]
- 5.Richardson MA, Post-White J, Singletary SE, Justice B. Recruitment for complementary/alternative medicine trials: who participates after breast cancer. Ann Behav Med 1998;20:190-8. [DOI] [PubMed]
- 6.Rogers EM. Diffusion of innovations. 4th ed. New York: Free Press; 1995.
- 7.Norman DA. Growing up: moving from technology-centered to human-centered products. In: The invisible computer. Cambridge: MIT Press; 1998. Available: mitpress.mit.edu/books/NORVH/chapter2.html (accessed 2003 Mar 31).
- 8.McCray AT, Ide NC. Design and implementation of a national clinical trials registry. J Am Med Inform Assoc 2000;7(3):313-23. [DOI] [PMC free article] [PubMed]
- 9.Manheimer E, Anderson D. Survey of public information about ongoing clinical trials funded by industry: evaluation of completeness and accessibility. BMJ 2002;325:528-31. [DOI] [PMC free article] [PubMed]
- 10.Dickersin K. How important is publication bias? A synthesis of available data. AIDS Educ Prev 1997;9(Suppl A):15-21. [PubMed]
- 11.Guidance for industry: information program on clinical trials for serious or life-threatening diseases and conditions. Washington: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research; 2002. Available: www.fda.gov/cder/guidance/4856fnl.htm (accessed 2003 Mar 31).
- 12.Eysenbach G, Jadad AR. Evidence-based patient choice and consumer health informatics in the Internet age. J Med Internet Res 2001;3(2):e19. Available: www.jmir.org/2001/2/e19 (accessed 2003 Mar 31). [DOI] [PMC free article] [PubMed]
- 13.Rehnquist J. Clinical trial Web sites: a promising tool to foster informed consent. Washington: Office of the Inspector General, US Department of Health and Human Services; 2002. Publ no OEI-01-97-00198. Available (PDF format): http://oig.hhs.gov/oei/reports/oei-01-97-00198.pdf (accessed 2003 Mar 31).
- 14.Jadad AR. Promoting partnerships: challenges for the Internet age. BMJ 1999;319:761-4. Available: www.bmj.com/cgi/content/full/319/7212/761 (accessed 2003 Mar 31). [DOI] [PMC free article] [PubMed]
- 15.Jadad AR, Enkin MW. The new alchemy: transmuting information to knowledge in the electronic age [editorial]. CMAJ 2000;162(13):1826-8. [PMC free article] [PubMed]


