Abstract
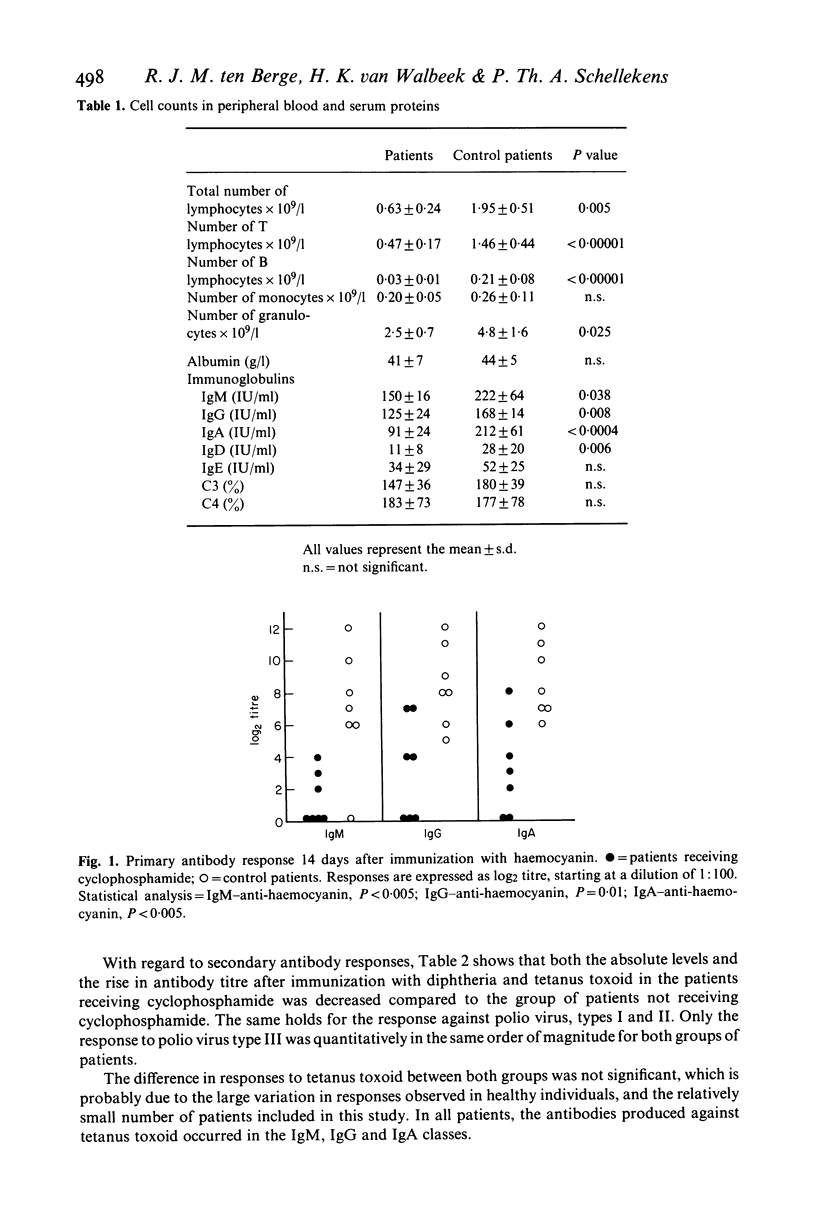
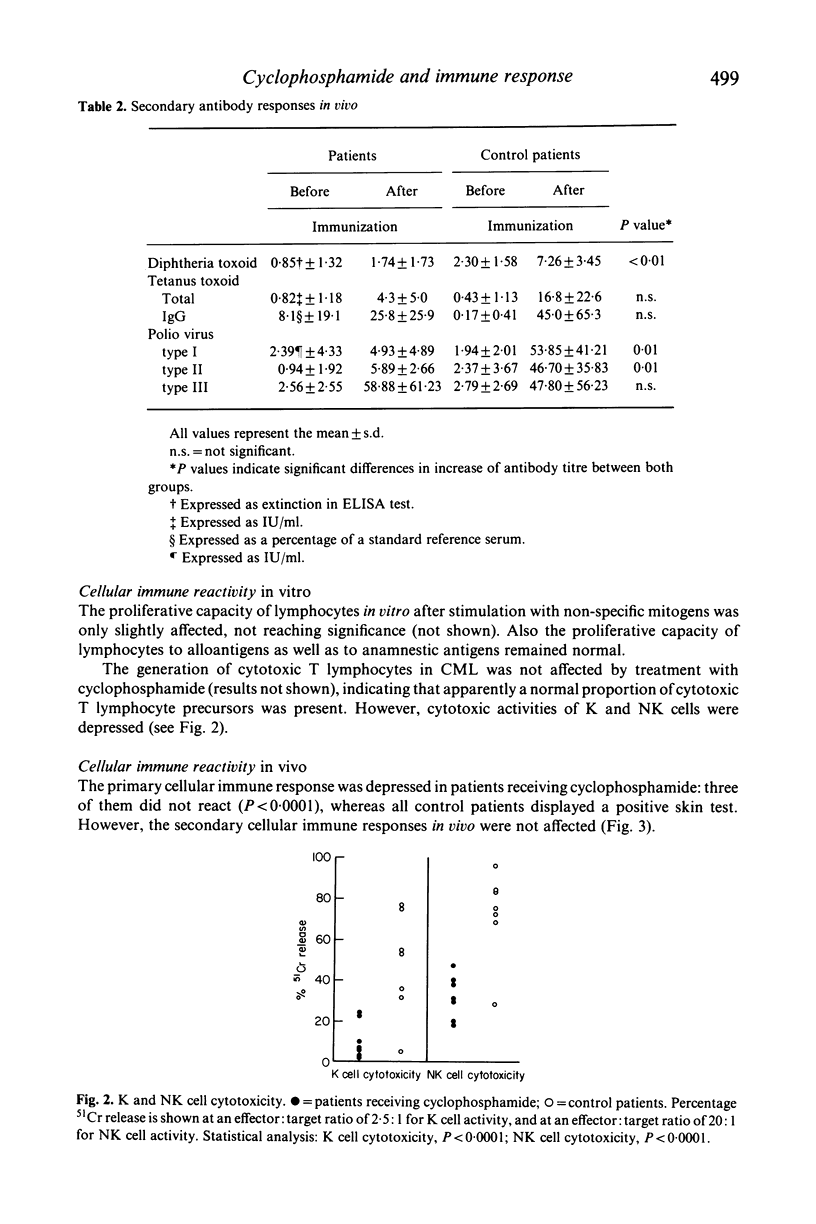
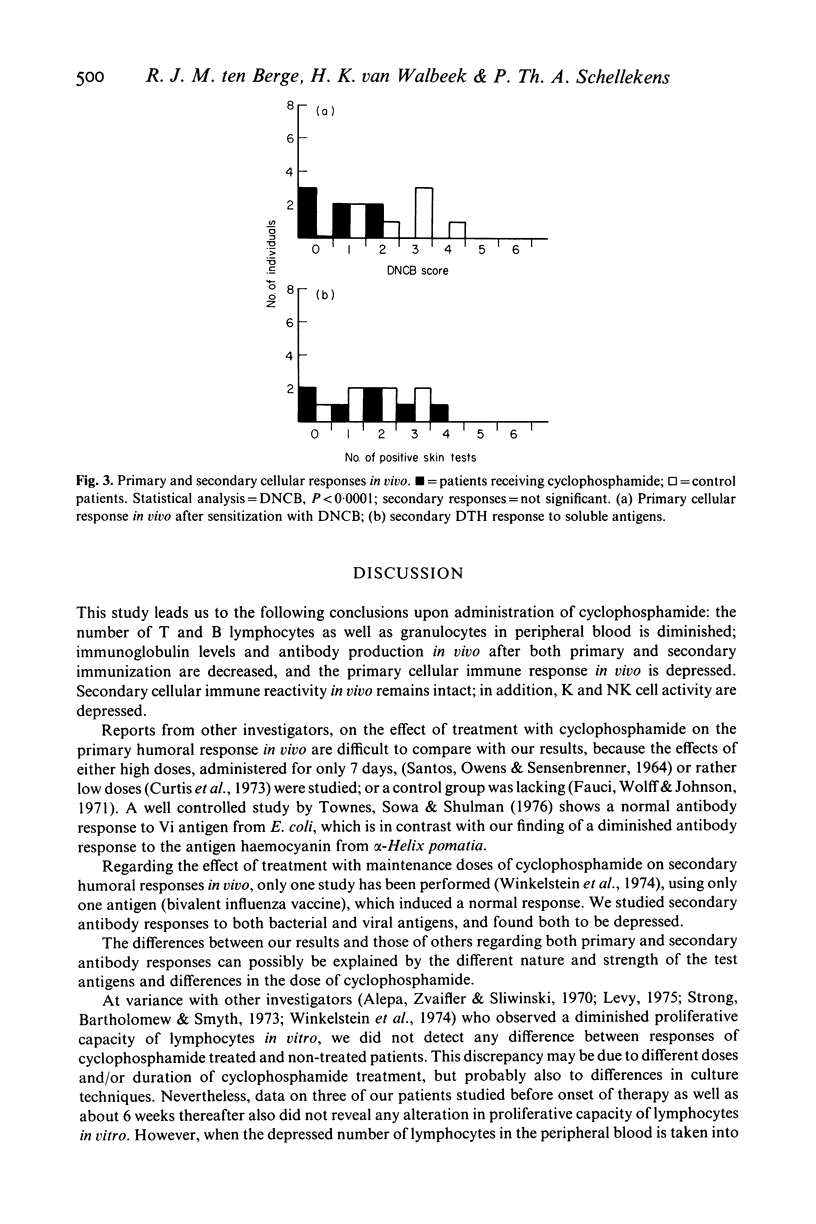
In a group of eight patients suffering from clinically definite multiple sclerosis, we studied the effects of treatment with cyclophosphamide on the immune reactivity in vitro and in vivo. The results are compared with those obtained in a control group consisting of eight patients who received no drug therapy and who were matched with the former group for age, sex and severity of disease. The results indicate that therapy with cyclophosphamide at a mean dose of 100 mg/day induces a profound lymphocytopenia in peripheral blood involving both T and B cells. Serum levels of immunoglobulins as well as primary and secondary antibody responses were depressed. In tests with standardized cell numbers, proliferative responses of lymphocytes in vitro and cytotoxic T cell function remained normal, whereas K and NK cell activities were diminished. Secondary cellular immune responses in vivo remained intact; however, the primary cellular immune response in vivo was markedly depressed. From these data, it is concluded that therapy with cyclophosphamide in man mainly affects humoral immune functions, but also cellular immunity, although to a lesser extent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alepa F. P., Zvaifler N. J., Sliwinski A. J. Immunologic effects of cyclophosphamide treatment in rheumatoid arthritis. Arthritis Rheum. 1970 Nov-Dec;13(6):754–760. doi: 10.1002/art.1780130604. [DOI] [PubMed] [Google Scholar]
- Astaldi A., Van de Griend R. J., Schellekens P. T., Vossen J. M. Are T gamma of myelomonocytic lineage? Eur J Immunol. 1982 Jun;12(6):527–530. doi: 10.1002/eji.1830120615. [DOI] [PubMed] [Google Scholar]
- Curtis J. E., Sharp J. T., Lidsky M. D., Hersh E. M. Immune response of patients with rheumatoid arthritis during cyclophosphamide treatment. Arthritis Rheum. 1973 Jan-Feb;16(1):34–42. doi: 10.1002/art.1780160106. [DOI] [PubMed] [Google Scholar]
- Dale D. C., Fauci A. S., Wolff S. M. The effect of cyclophosphamide on leukocyte kinetics and susceptibility to infection in patients with Wegener's granulomatosis. Arthritis Rheum. 1973 Sep-Oct;16(5):657–664. doi: 10.1002/art.1780160510. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Wolff S. M., Johnson J. S. Effect of cyclophosphamide upon the immune response in Wegener's granulomatosis. N Engl J Med. 1971 Dec 30;285(27):1493–1496. doi: 10.1056/NEJM197112302852701. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Goetzl E. J., Steinberg A. D. Cyclophosphamide: use in practice. Ann Intern Med. 1974 Apr;80(4):531–540. doi: 10.7326/0003-4819-80-4-531. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURTZKE J. F. FURTHER NOTES ON DISABILITY EVALUATION IN MULTIPLE SCLEROSIS, WITH SCALE MODIFICATIONS. Neurology. 1965 Jul;15:654–661. doi: 10.1212/wnl.15.7.654. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Oldham R. K., Cannon G. C., Herberman R. B. Specificity of natural cytotoxic reactivity of normal human lymphocytes against a myeloid leukemia cell line. J Natl Cancer Inst. 1977 Jul;59(1):77–82. doi: 10.1093/jnci/59.1.77. [DOI] [PubMed] [Google Scholar]
- SANTOS G. W., OWENS A. H., Jr, SENSENBRENNER L. L. EFFECTS OF SELECTED CYTOTOXIC AGENTS ON ANTIBODY PRODUCTION IN MAN; A PRELIMARY REPORT. Ann N Y Acad Sci. 1964 Mar 31;114:404–423. doi: 10.1111/j.1749-6632.1964.tb53594.x. [DOI] [PubMed] [Google Scholar]
- Strong J. S., Bartholomew B. A., Smyth C. J. Immunoresponsiveness of patients with rheumatoid arthritis receiving cyclophosphamide or gold salts. Ann Rheum Dis. 1973 May;32(3):233–237. doi: 10.1136/ard.32.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes A. S., Sowa J. M., Shulman L. E. Controlled trial of cyclophosphamide in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):563–573. doi: 10.1002/art.1780190308. [DOI] [PubMed] [Google Scholar]
- Winkelstein A., Ruben R. L., Tolchin S. F., Pollock B. H. Mechanisms of immunosuppression: effect of cyclophosphamide on responses to influenza immunization. J Lab Clin Med. 1974 Mar;83(3):504–510. [PubMed] [Google Scholar]
- Zeijlemaker W. P., Roos M. T., Schellekens P. T., Eijsvoogel V. P. Antibody-dependent human lymphocytotoxicity: a micro assay system. Eur J Immunol. 1975 Aug;5(8):579–584. doi: 10.1002/eji.1830050815. [DOI] [PubMed] [Google Scholar]
- Zeijlemaker W. P., Van Oers M. H., Eijsvoogel V. P. Human lymphocyte subpopulations involved in MLC and CML. Scand J Immunol. 1976 Jun;Suppl 5:143–156. doi: 10.1111/j.1365-3083.1976.tb03865.x. [DOI] [PubMed] [Google Scholar]


