Abstract
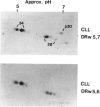
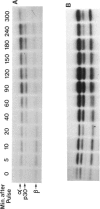
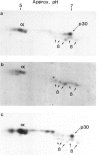
A large series of leukaemias (1,512 cases) and leukaemic cell lines (40) have been tested for selective expression of a monomorphic HLR-DR determinant using a monoclonal antibody (DA2). Relatively mature myeloid leukaemias (APML, CGL) and erythroid leukemias are DR-, in contrast to most (72% leukaemias of myeloid precursors (e.g. AML) which are DR+. Non-T ALL are DR+ but T (thymic) ALL are invariably DR-. In contrast to the latter, some leukaemias with mature T cell phenotypes are DR+. Leukaemias or lymphomas of B cells and B cell precursors (e.g. pre-BALL) are invariably DR+, whereas myeloma or plasma cell leukaemias are DR-. This pattern of selective expression appears to closely parallel that seen in normal haemopoietic differentiation. Biochemical features of HLA-DR structures on leukaemic cells have been compared with the known features of B cell derived DR molecules and in one case ALL compared with an autologous (EBV transformed) B cell line. Most leukemic cells showed the same general alpha and beta two chain structure. However, B cell line and most chronic leukaemias showed the presence of an extra band of molecular weight 30,000 daltons (p30) with an intermediate electrophoretic mobility on SDS-PAGE between that of the alpha and beta DR chains. In acute leukaemias and leukaemic cell lines (i.e. immature cells) p30 was not seen unless short labelling times were used. Two dimensional NEPHGE/SDS-PAGE under appropriate labelling conditions showed that the pattern of spots obtained from an ALL line (Nalm-6) and its autologous EBV transformed partner (B85) were similar though not identical. Pulse chase labelling of Nalm-6 and B85 showed that the turnover rate of p30 relative to DR alpha and beta chains, differed in the two lines.
Full text
PDF

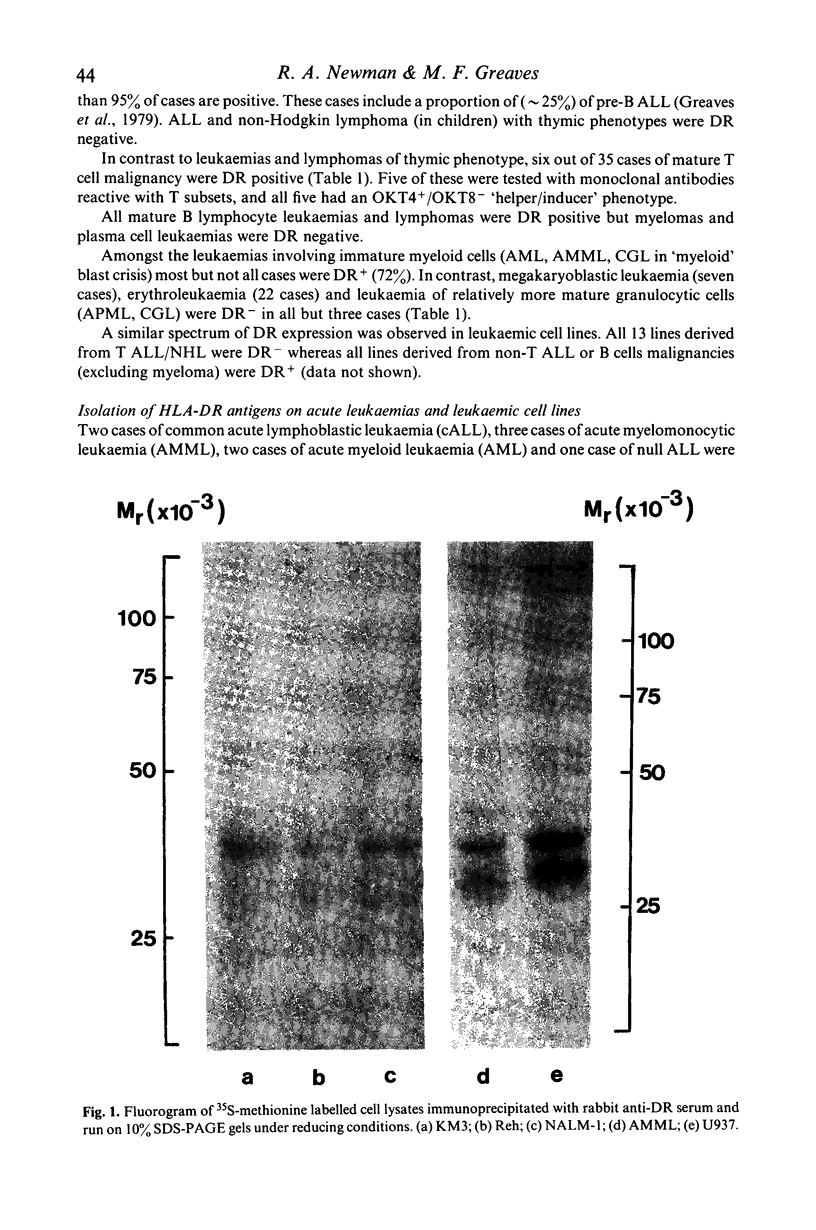


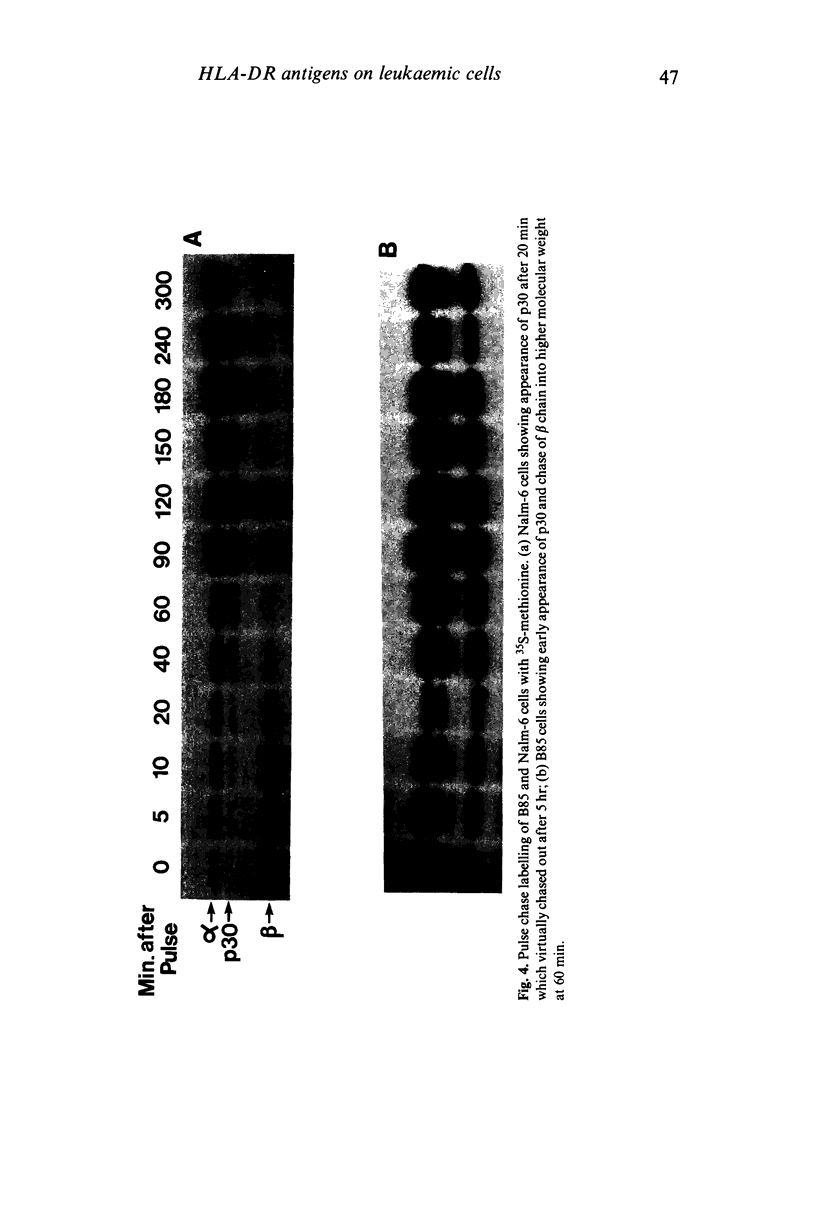
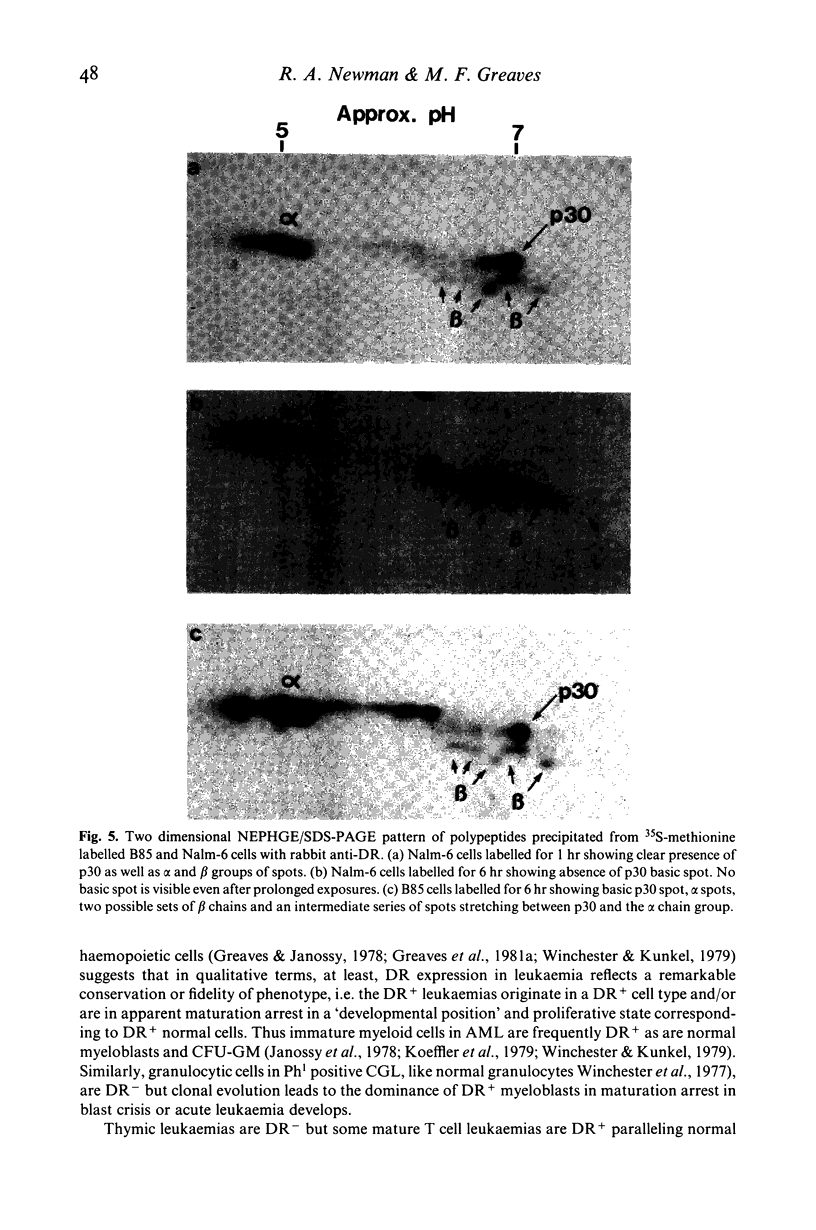


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Characterization of HLA-D-region antigens by two-dimensional gel electrophoresis. Molecular-genotyping. J Exp Med. 1980 Aug 1;152(2 Pt 2):18s–36s. [PubMed] [Google Scholar]
- Greaves M. F., Delia D., Robinson J., Sutherland R., Newman R. Exploitation of monoclonal antibodies: a "who's who" of haemopoietic malignancy. Blood Cells. 1981;7(2):257–280. [PubMed] [Google Scholar]
- Greaves M. F., Janossy G., Peto J., Kay H. Immunologically defined subclasses of acute lymphoblastic leukaemia in children: their relationship to presentation features and prognosis. Br J Haematol. 1981 Jun;48(2):179–197. [PubMed] [Google Scholar]
- Greaves M. F., Rao J., Hariri G., Verbi W., Catovsky D., Kung P., Goldstein G. Phenotypic heterogeneity and cellular origins of T cell malignancies. Leuk Res. 1981;5(4-5):281–299. doi: 10.1016/0145-2126(81)90001-1. [DOI] [PubMed] [Google Scholar]
- Greaves M. Cell surface structures, differentiation and malignancy in the haemopoietic system. Symp Soc Exp Biol. 1978;32:429–442. [PubMed] [Google Scholar]
- Greaves M., Janossy G. Patterns of gene expression and the cellular origins of human leukaemias. Biochim Biophys Acta. 1978 Oct 27;516(2):193–230. doi: 10.1016/0304-419x(78)90008-2. [DOI] [PubMed] [Google Scholar]
- Greaves M., Verbi W., Vogler L., Cooper M., Ellis R., Ganeshaguru K., Hoffbrand V., Janossy G., Bollum F. J. Antigenic and enzymatic phenotypes of the pre-B subclass of acute lymphoblastic leukaemia. Leuk Res. 1979;3(6):353–362. doi: 10.1016/0145-2126(79)90032-8. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Francis G. E., Capellaro D., Goldstone A. H., Greaves M. F. Cell sorter analysis of leukaemia-associated antigens on human myeloid precursors. Nature. 1978 Nov 9;276(5684):176–178. doi: 10.1038/276176a0. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Niskanen E., Cline M., Billing R., Golde D. Human myeloid precursors forming colonies in diffusion chambers expresses the Ia-like antigen. Blood. 1979 Nov;54(5):1188–1191. [PubMed] [Google Scholar]
- McMillan M., Frelinger J. A., Jones P. P., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens. Two dimensional electrophoretic analyses and high pressure liquid chromatography tryptic peptide maps of products of the I-A and I-E subregions and of an associated invariant polypeptide. J Exp Med. 1981 Apr 1;153(4):936–950. doi: 10.1084/jem.153.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Janossy G., Greaves M. F., Tsubota T., Srivastava B. I., Morikawa S., Tatsumi E. Expression of an antigen associated with acute lymphoblastic leukemia in human leukemia-lymphoma cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1269–1277. doi: 10.1093/jnci/60.6.1269. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Sutherland R., Greaves M. F. The biochemical characterization of a cell surface antigen associated with acute lymphoblastic leukemia and lymphocyte precursors. J Immunol. 1981 May;126(5):2024–2030. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Kissonerghis A. M., Lodish H. F. Biosynthesis of HLA-A and HLA-B antigens in vivo. J Biol Chem. 1980 Oct 25;255(20):9678–9684. [PubMed] [Google Scholar]
- Schlossman S. F., Chess L., Humphreys R. E., Strominger J. L. Distribution of Ia-like molecules on the surface of normal and leukemic human cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1288–1292. doi: 10.1073/pnas.73.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snary D., Barnstable C. J., Bodmer W. F., Goodfellow P. N., Crumpton M. J. Cellular distrubtion, purification, and molecular nature of human Ia antigens. Scand J Immunol. 1977;6(5):439–452. doi: 10.1111/j.1365-3083.1977.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Kaufman J. F., Terhorst C., Strominger J. L. Purification and structural characterisation of human HLA-linked B-cell antigens. Nature. 1977 Jul 21;268(5617):213–218. doi: 10.1038/268213a0. [DOI] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Kunkel H. G. The human Ia system. Adv Immunol. 1979;28:221–292. [PubMed] [Google Scholar]
- Winchester R. J., Ross G. D., Jarowski C. I., Wang C. Y., Halper J., Broxmeyer H. E. Expression of Ia-like antigen molecules on human granulocytes during early phases of differentiation. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4012–4016. doi: 10.1073/pnas.74.9.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kretser T. A., Crumpton M. J., Bodmer J. G., Bodmer W. F. Demonstration of two distinct light chains in HLA-DR-associated antigens by two-dimensional gel electrophoresis. Eur J Immunol. 1982 Mar;12(3):214–221. doi: 10.1002/eji.1830120309. [DOI] [PubMed] [Google Scholar]