Infliximab (Remicade) is a chimeric (part human, part mouse) antibody that targets tumour necrosis factor-α (TNF-α), a potent proinflammatory cytokine implicated in different inflammatory diseases, such as Crohn's disease and rheumatoid arthritis.1,2 (Cytokines are molecules secreted from cells. Among other things, they play an important role in interactions, modulations and regulation of the immune system. Thus, they play a part in the reactions that cause inflammation and killing of invading microbial agents, including tuberculosis (TB). The cytokines include interleukins, TNF-α and interferon-γ.) Although the role of TNF-α in the human immune response to mycobacteria is incompletely understood, in animal models TNF-α plays a central role in the formation of granulomata and containment of disease (Fig. 1).3,4
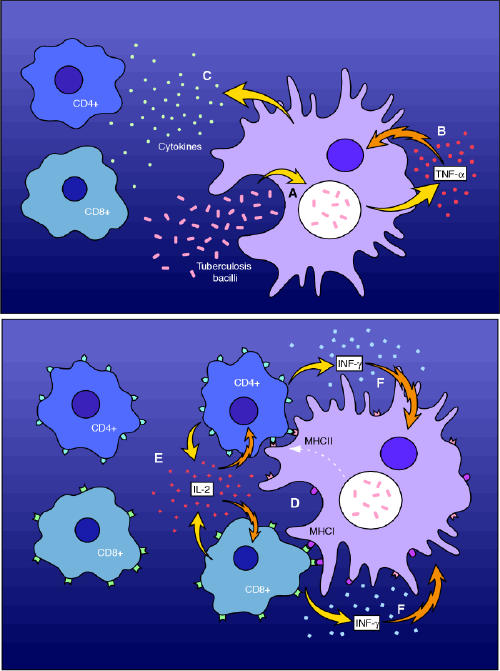
Fig. 1: The putative role of tumour necrosis factor-α (TNF-α) in the cell-mediated normal human immune response to tuberculosis infection. The macrophage (A) phagocytoses the invading mycobacteria. This results in the release of TNF-α (B) and other cytokines (C), the effect of which is further activation of cell-mediated immunity. The early release of TNF-α enhances the ability of macrophages to phagocytose and kill mycobacteria. Antigen presentation through major histocompatibility complexes (MHC) leads to the release (D) of other cytokines (interleukin-2) with further recruitment of T lymphocytes (E). Lastly, T-lymphocyte release of interferon-γ (F) further activates the macrophage to enhance bacterial killing. Inhibitors of TNF-α such as infliximab interfere with this process at an early stage (B). Photo: Myra Rudakewich
There are now a large number of reports of TB in close temporal association with the initiation of TNF-α inhibitors and an increased rate of TB among patients treated with infliximab, as compared with available data on background rates.5,6,7 Although passive surveillance data do not prove a causal relationship between infliximab and TB (e.g., increased awareness alone could be contributing to diagnoses of TB independent of infliximab therapy), the association is not thought to be coincidental.5 In most instances, TB appears to be secondary to reactivation of latent TB infection.
In Canada, infliximab is approved for use in the treatment of Crohn's disease or rheumatoid arthritis that is not responding to other anti- inflammatory agents.1,8,9,10 Etanercept (Enbrel), a recombinant TNF receptor fusion protein, also targets TNF-α, but is only approved for use in patients with rheumatoid arthritis.11,12,13 Neither drug is curative nor currently approved for use in chronic inflammatory conditions other than Crohn's disease and rheumatoid arthritis. Infliximab and etanercept are expensive, which accounts for their current omission from most drug benefit lists or regional formularies.
Although clinical and epidemiological reports are preliminary, there is nonetheless general agreement that patients who are being considered for treatment with infliximab should be screened for active TB and latent TB infection before the introduction of the agent (Box 1).13,14,15,16 It is recommended that patients with proven active disease complete a satisfactory course of antituberculosis drug treatment before infliximab is introduced.5,14
Box 1.

Screening for TB in patients with rheumatoid arthritis may be challenging, because the clinical and radiological features of rheumatoid lung disease may overlap with those of TB. Likewise, virtually all of the clinical and radiological features of Crohn's disease are indistinguishable from those of ileocecal TB. A diagnosis of Crohn's disease, especially in patients who are Aboriginal or were born in countries where TB is endemic,17 should always raise suspicion of ileocecal TB.7
Most guidelines for the treatment of latent TB infection recommend that when the pretest probability of a true-positive tuberculin skin test is high, and the risk of reactivation TB is high, then a Mantoux test cut-off point of ≥ 5 mm or more should be indicative of latent TB infection.18 When the risk of reactivation is judged to be extraordinarily high (for example in people with HIV/AIDS), then a ≥ 5-mm cut-off point is used regardless of the pretest probability of a true-positive tuberculin skin test.18 Whether infliximab constitutes such an extraordinarily high risk has not been established yet. A conservative approach would be to assume that it does. Routine anergy testing is not recommended.
The management of latent TB infection in candidates for infliximab is controversial and likely to remain so until new information concerning the risk of reactivation in recipients of the agent is available (Box 2). The controversy surrounds the question of whether, in the interest of TB prevention, it is necessary to complete preventive therapy before the introduction of infliximab, or whether it is sufficient to simply initiate treatment of latent TB infection before the introduction of infliximab. Implicit in the first position is the withholding of infliximab for the 9 months that are necessary to complete isoniazid preventive therapy. People with latent TB infection are understood to be harbouring fewer than 100 000 tubercle bacilli.19 To completely destroy this population of bacilli requires 9 months of isoniazid. Implicit in the second position is the understanding that infliximab may be safely introduced 1 day after the start of preventive therapy. The available clinical literature5,6,7 advocates for the first position, imputing to infliximab a degree of acute, electively induced immunosuppression that might result in TB reactivation in the absence of a complete course of preventive therapy.
Box 2.

Those arguing in favour of the second position may cite evidence of isoniazid's efficacy in treating latent TB infection in patients with HIV/AIDS,20 asserting that infliximab-induced immunosuppression can be no worse than HIV/AIDS-induced immunosuppression (single-dose therapy with infliximab is not associated with changes in major circulating lymphocyte populations21). However, the circumstances may not be the same. HIV/AIDS immunosuppression is virally induced and very slowly progressive. At the point of introduction of preventive therapy the patient has not, despite the existing immunosuppression, developed active TB. Deferring a recommendation of treatment of latent TB infection in such a patient is usually not an option. Infliximab immunosuppression, on the other hand, is electively drug induced. Transiently, it may be very potent. Deferring the onset of the immunosuppressed state may be an option, though understandably unattractive from the point of view of management of a patient's Crohn's disease or rheumatoid arthritis. Newer and shorter-course treatments of latent TB infection may reduce waiting times to the completion of preventive therapy.18
The benefits of accessing a TNF-α inhibitor may outweigh the risks of hepatotoxicity from TB-preventive therapy. Although the incidence of rheumatoid arthritis13 and the risk of isoniazid-related hepatitis22 increase with age, the risk to patients with rheumatoid arthritis of isoniazid–induced hepatitis is not prohibitive. Similarly, though hepatobiliary abnormalities may be associated with Crohn's disease,23 they may not of themselves preclude the use of isoniazid. Under ordinary circumstances, older age alone or pre-existing liver disease, or both, do not represent absolute contraindications to the judicious use of isoniazid. They do, however, dictate the need for careful and systematic monitoring of symptoms and aminotransferase levels. A history of pre-existing liver disease should be sought and baseline aminotransferase levels should be measured before the introduction of isoniazid. Once isoniazid is introduced, patients should be followed for symptoms of hepatotoxicity; those at increased risk of hepatotoxicity, such as patients with pre-existing liver disease or alcohol addiction or individuals who are already receiving a hepatotoxic drug, should have serial measurements of aminotransferase levels. If symptoms of hepatitis occur or aminotransferase levels exceed 3–5 times the upper limit of normal, then isoniazid should be withheld.
The half-life of infliximab is 10 days,24 and its biological effect persists for up to 2 months. Individuals being treated with infliximab who have close contact with patients who have infectious TB should be treated for presumptive latent TB infection. If the TNF-α inhibitor is continued or believed to be active for more than 8–12 weeks after final contact with the TB source case, then preventive therapy should be continued, even when a repeat tuberculin skin test at 8–12 weeks is not indicative of latent infection. Orme and Cooper have proposed that TNF plays a major role in triggering or amplifying the chemokine-driven processes that are central to delayed-type hypersensitivity and the tuberculin skin test.25 Although this suggests that infliximab could cause a false-negative tuberculin skin test, there is anecdotal evidence that delayed-type hypersensitivity is preserved after infliximab.26 At the moment, it is not established whether TNF-α inhibition itself will result in a false-negative tuberculin skin test.
During therapy and for a period of at least 6 months post infusion (complete elimination of infliximab may require up to 6 months),27,28,29 health care workers who look after patients being treated with infliximab should maintain a high level of suspicion for TB. TNF-α is believed to be responsible for some of the clinical manifestations of TB, including weight loss, night sweats and tissue destruction.30 Accordingly, its inhibition by infliximab may mask some of the usual signs and symptoms of the disease.
Evidence implicating etanercept, the other currently available TNF-α inhibitor, with TB reactivation is less compelling,31 perhaps because of differential effects of the 2 agents on monocytes and lymphocytes,4,32 or perhaps because it was studied in a different patient population.5 Nonetheless, until more information is available to suggest otherwise, it would be prudent to consider the preceding comments concerning infliximab to be equally applicable to etanercept.
In summary, physicians who prescribe these biological agents need to screen patients for TB and latent TB infection and treat each of these conditions appropriately. Health care providers also need to be vigilant for signs and symptoms of TB among patients with Crohn's disease and rheumatoid arthritis who are taking TNF-α inhibitors.
Key points .
The reported frequency of TB in association with infliximab therapy is much higher than the reported frequency of other opportunistic infections associated with the drug.
TB tends to occur after a median of 12 weeks of infliximab therapy.
Infliximab-related TB frequently manifests as extrapulmonary disease, particularly disseminated disease.
Many patients with infliximab-related TB are taking concurrent corticosteroids or other immunosuppressants suggesting a possible additive or synergistic effect of infliximab.
Infliximab may be more potent than other therapeutic agents capable of reactivating latent TB infection.
Acknowledgments
We thank Drs. Dick Menzies, Vernon Hoeppner, Dennis Kunimoto, Maria Gutschi, Anthony Russell and Richard Fedorak for their review of the manuscript and Susan Falconer for her preparation of the manuscript.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Long prepared the initial draft of the article. Dr. Gardam critically reviewed and revised the article and prepared the initial draft of the figure. Both authors approved the submitted version of the article.
Competing interests: None declared for Dr. Long. Dr. Gardam has received 2 speaker's fees and travel assistance for educating rheumatologists about TB and TB skin testing from Schering.
Correspondence to: Dr. Richard Long, Department of Medicine, University of Alberta Hospitals, Rm. 2E4.21, Walter Mackenzie Centre, 8440 112 St., Edmonton AB T6G 2B7; fax 780 407-6384; richard.long@ualberta.ca
References
- 1.Canadian Pharmacists Association. Remicade [product monograph]. Compendium of pharmaceuticals and specialties. Ottawa: The Association; 2002. p. 1443-5.
- 2.Feldmann M, Charles P, Taylor P, Maini RN. Biologic insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol 1998;20:211-28. [DOI] [PubMed]
- 3.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, Tsang E, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun 2001;69:1847-55. [DOI] [PMC free article] [PubMed]
- 4.Van Deventer SJH. Transmembrane TNF-α, induction of apoptosis, and the efficacy of TNF-targeting therapies in Crohn's disease [editorial]. Gastroenterology 2001;121:1242-6. [DOI] [PubMed]
- 5.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman W, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med 2001;345:1098-103. [DOI] [PubMed]
- 6.Tuberculosis and treatment with infliximab (correspondence). N Engl J Med 2002;346:623-6. [PubMed]
- 7.Wagner TE, Huseby ES, Huseby JS. Exacerbation of mycobacterium tuberculosis enteritis masquerading as Crohn's disease after treatment with a tumor necrosis factor-alpha inhibitor. Am J Med 2002;112:67-9. [DOI] [PubMed]
- 8.Panaccione R, for the Canadian Consensus Group on the Use of Infliximab in Crohn's disease. Infliximab for the treatment of Crohn's disease: review and indications for clinical use in Canada. Can J Gastroenterol 2001;15:371-5. [DOI] [PubMed]
- 9.Freeman HJ. Infliximab for Crohn's disease: more questions than answers. Can J Gastroenterol 2001;15:360-2. [DOI] [PubMed]
- 10.Brzezinski A. Progress in treatment for Crohn's disease [editorial]. Am J Gastroenterol 2001;96:626-7. [DOI] [PubMed]
- 11.Canadian Pharmacists Association. Enbrel [product monograph]. Compendium of pharmaceuticals and specialties. Ottawa: The Association; 2002. p. 569-72.
- 12.Klippel JH. Biologic therapy for rheumatoid arthritis. N Engl J Med 2000; 343: 1640-1. [DOI] [PubMed]
- 13.Pisetsky DS, St. Clair EW. Progress in the treatment of rheumatoid arthritis. JAMA 2001;286:2787-90. [DOI] [PubMed]
- 14.Fedorak RN. Canadian Association of Gastroenterology clinical practice guidelines: the use of infliximab in Crohn's disease. Can J Gastroenterol 2001;15(6):367-70. [DOI] [PubMed]
- 15.Menzies D, Pourier L. Diagnosis of tuberculosis infection and disease. In: Long R, editor. The Canadian tuberculosis standards. 5th ed. Ottawa: Health Canada and the Canadian Lung Association; 2000. p. 45-65.
- 16.Njoo H, Long R. The epidemiology of tuberculosis in Canada. In: Long R, editor. The Canadian tuberculosis standards. 5th ed. Ottawa: Health Canada and the Canadian Lung Association; 2000. p. 3-14.
- 17.Palmer KR, Patil DH, Basran GS, Riordan JF, Silk DBA. Abdominal tuberculosis in urban Britain — a common disease. Gut 1985;26:1296-305. [DOI] [PMC free article] [PubMed]
- 18.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161(Suppl):S221-247.10764341
- 19.Bates JH. The tuberculin skin test and preventive treatment of tuberculosis. In: Rom WN, Garey S, editors. Tuberculosis. New York: Little Brown; 1996. p. 865-71.
- 20.Pope JW, Jean SS, Ho HL, Hafner A, Johnson WD. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet 1993;342(8866):268-72. [DOI] [PubMed]
- 21.Meenan J, Hommes DW, van Dulleman H, Obradov D, Shealy DJ, Tytgat GNJ, et al. The influence of TNFα mAb, cA2, on circulating lymphocyte populations. Gastroenterology 1997;112(Suppl):A1039.
- 22.Kopanoff DE, Snider DE, Caras GJ. Isoniazid related hepatitis. Am Rev Respir Dis 1978;117:991-1001. [DOI] [PubMed]
- 23.Danzi JT. Extraintestinal manifestations of idiopathic inflammatory bowel diseases. Arch Intern Med 1988;148:297-302. [PubMed]
- 24.Infliximab (Remicade) for Crohn's disease. Med Lett Drugs Ther 1999;4:19-20. [PubMed]
- 25.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today 1999;20(7):307-12. [DOI] [PubMed]
- 26.Feldmann M, Elliott MJ, Woody JN, Maini RN. Anti-tumor necrosis factor-α therapy of rheumatoid arthritis. Adv Immunol 1997;64:283-350. [DOI] [PubMed]
- 27.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340:1398-405. [DOI] [PubMed]
- 28.Rutgeerts P, D'Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology 1999;117:761-9. [DOI] [PubMed]
- 29.Maini RN, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumor necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet 1999;354:1932-9. [DOI] [PubMed]
- 30.Rook GA, Taverne J, Leveton C, Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology 1987;62:229-34. [PMC free article] [PubMed]
- 31.Cush JJ, Matteson EL. FDA Advisory Committee reviews safety of TNF-α inhibitors. ACR Hotline 2001 Sept. Available: www.rheumatology.org/research/hotline/0901tnf.html (accessed 2003 Mar 14).
- 32.Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2001;121:1088-94. [DOI] [PubMed]


