Abstract
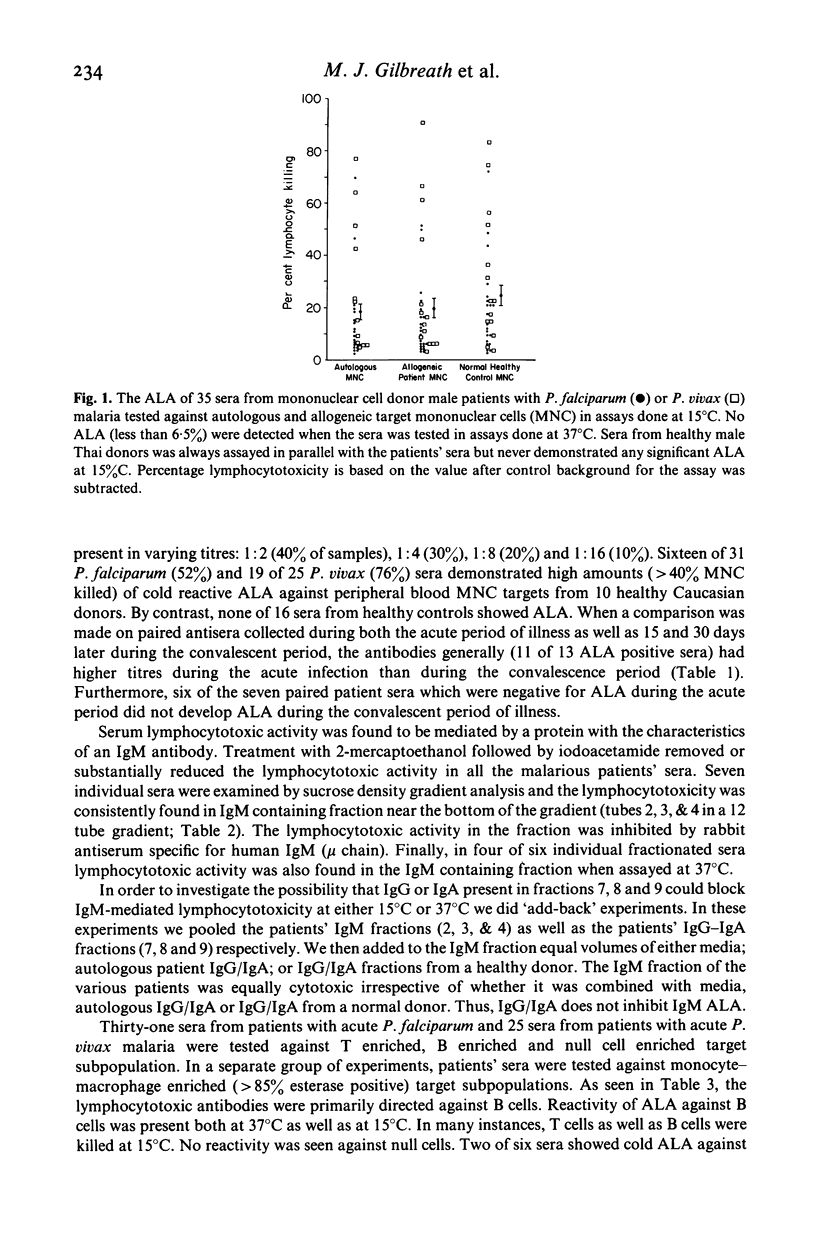
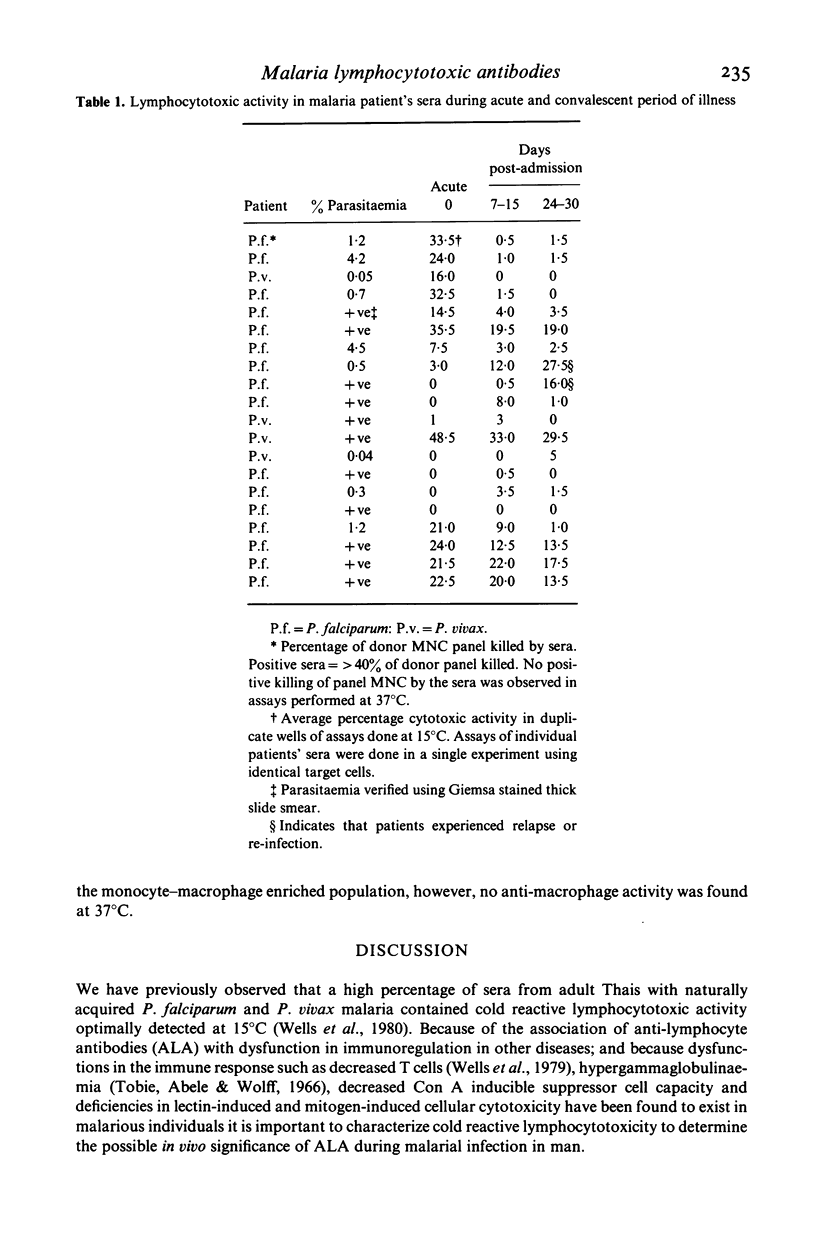
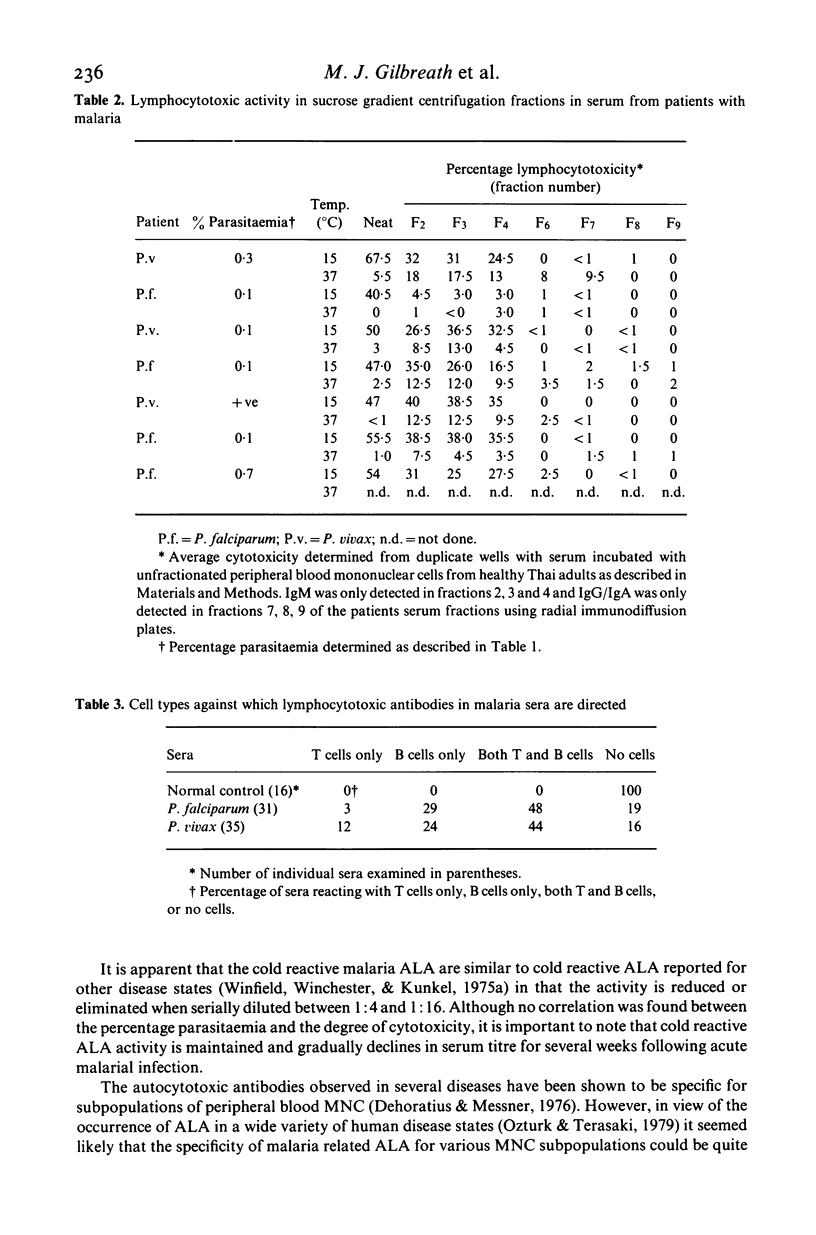
Characterization of cold reactive lymphocytotoxic antibodies present in sera from Thai adults with malaria revealed that the antibodies are predominantly 19S (IgM), directed against both autologous and allogeneic mononuclear cells, complement-dependent, present in titres ranging from 1:2 to 1:16, and exhibit greater lymphocytotoxic activity during the acute stage of malarial infection than during the convalescent stage. The lymphocytotoxic antibodies were primarily directed against B cell targets or both B as well as T cell targets. In addition some sera were reactive with enriched monocyte/macrophage indicator cells at 15 degrees C, but not 37 degrees C. Antibodies directed against B cell targets were lymphocytotoxic both at 15 degrees C as well as 37 degrees C. The results indicate that IgM lymphocytotoxic antibodies in the sera of patients with malaria are directed primarily against B cells with reactivity to a lesser extent against T cells and macrophages and thus may play an immunoregulatory function in the humoral immune response to malaria infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cicciarelli J. C., Chia D., Terasaki P. I., Barnett E. V., Shirahama S. Human IgM anti-IgM cytotoxin for B lymphocytes. Tissue Antigens. 1980 Mar;15(3):275–282. doi: 10.1111/j.1399-0039.1980.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Dehoratius R. J., Henderson C., Strickland R. G. Lymphocytotoxins in acute and chronic hepatitis. Characterization and relationship to changes in circulating T lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):21–27. [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. S., Cunningham J. E., Terasaki P. I. Lymphocytotoxins and pernicious anemia. Blood. 1972 Jun;39(6):862–865. [PubMed] [Google Scholar]
- Kreisler M. J., Hirata A. A., Terasaki P. I. Cytotoxins in disease. 3. Antibodies against lymphocytes produced by vaccination. Transplantation. 1970 Nov;10(5):411–415. [PubMed] [Google Scholar]
- Lies R. B., Messner R. P., Williams R. C., Jr Relative T-cell specificity of lymphocytotoxins from patients with systemic lupus erythematosus. Arthritis Rheum. 1973 May-Jun;16(3):369–375. doi: 10.1002/art.1780160312. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Stacey M. C. Further characterization of the human autologous mixed leukocyte reaction (MLR). J Immunol. 1981 Feb;126(2):729–734. [PubMed] [Google Scholar]
- MacDermott R. P., Wells R. A., Zolyomi S., Pavanand K., Phisphumvidhi P., Permpanich B., Gilbreath M. Examination of peripheral blood mononuclear cells and sera from Thai adults naturally infected with malaria in assays of blastogenic responsiveness to mitogenic lectins and allogeneic cell surface antigens. Infect Immun. 1980 Dec;30(3):781–785. doi: 10.1128/iai.30.3.781-785.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Mickey M. R., Hirata A., Terasaki P. I. Autolymphocytotoxins following immunization by pregnancy, transplantation, and disease. Tissue Antigens. 1971;1(5):219–228. doi: 10.1111/j.1399-0039.1971.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Nisonoff A., Bangasser S. A. Immunological suppression of idiotypic specificities. Transplant Rev. 1975;27:100–134. doi: 10.1111/j.1600-065x.1975.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Ozturk G., Terasaki P. I. Non-HLA lymphocyte cytotoxins in various diseases. Tissue Antigens. 1979 Jul;14(1):52–58. doi: 10.1111/j.1399-0039.1979.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Park M. S., Terasaki P. I., Bernoco D. Autoantibody against B lymphocytes. Lancet. 1977 Sep 3;2(8036):465–467. doi: 10.1016/s0140-6736(77)91598-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y. J. Autoimmune and polyclonal B cell responses during murine malaria. Nature. 1978 Jul 13;274(5667):170–172. doi: 10.1038/274170a0. [DOI] [PubMed] [Google Scholar]
- Tobie J. E., Abele D. C., Wolff S. M., Contacos P. G., Evans C. B. Serum immunoglobulin levels in human malaria and their relationship to antibody production. J Immunol. 1966 Oct;97(4):498–505. [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., MacDermott R. P. Loss of circulating T lymphocytes with normal levels of B and 'null' lymphocytes in Thai adults with malaria. Clin Exp Immunol. 1979 Feb;35(2):202–209. [PMC free article] [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., Macdermott R. P. Anti-lymphocytotoxic antibodies in sera of Thai adults infected with Plasmodium falciparum or Plasmodium vivax. Clin Exp Immunol. 1980 Mar;39(3):663–667. [PMC free article] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Kunkel H. G. Association of cold-reactive antilymphocyte antibodies with lymphopenia in systemic lupus erythematosus. Arthritis Rheum. 1975 Nov-Dec;18(6):587–594. doi: 10.1002/art.1780180609. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Winchester R. J., Wernet P., Fu S. M., Kunkel H. G. Nature of cold-reactive antibodies to lymphocyte surface determinants in systemic lupus erythematosus. Arthritis Rheum. 1975 Jan-Feb;18(1):1–8. doi: 10.1002/art.1780180101. [DOI] [PubMed] [Google Scholar]


