Abstract
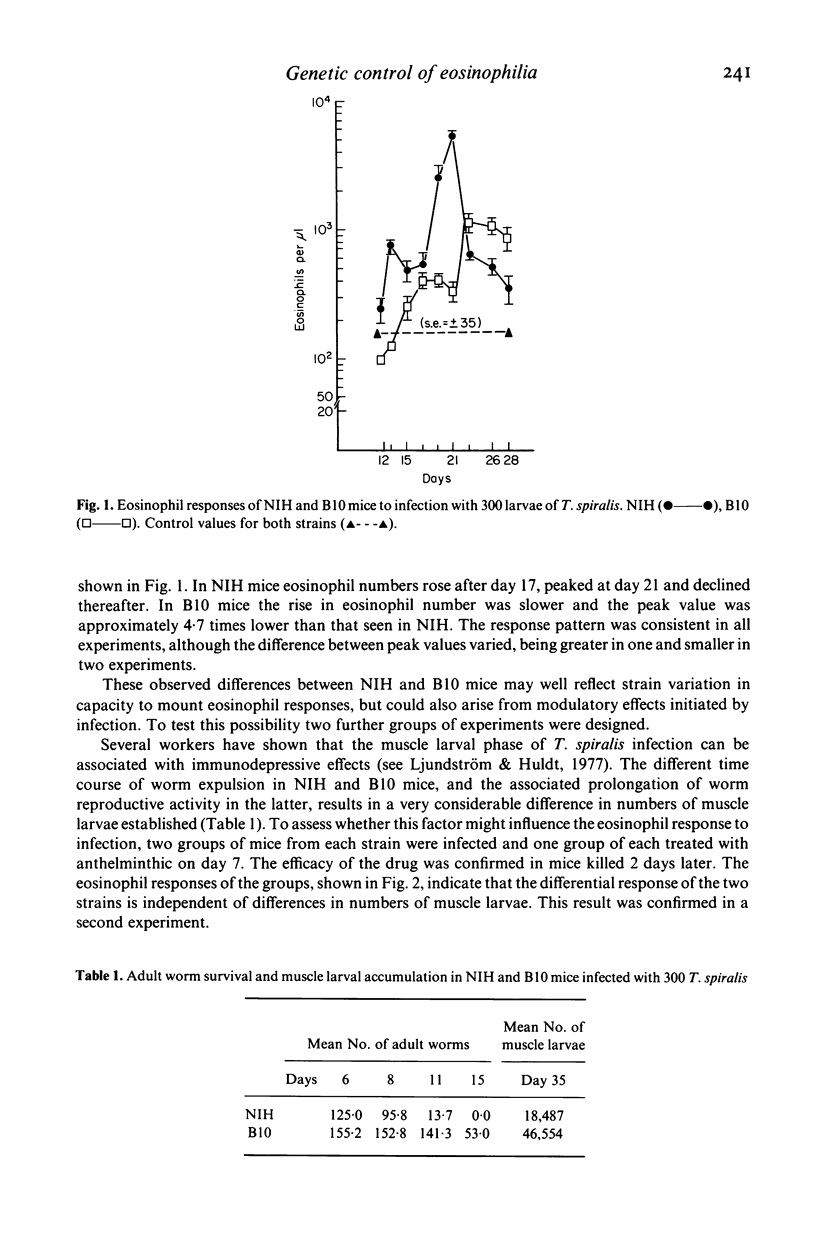
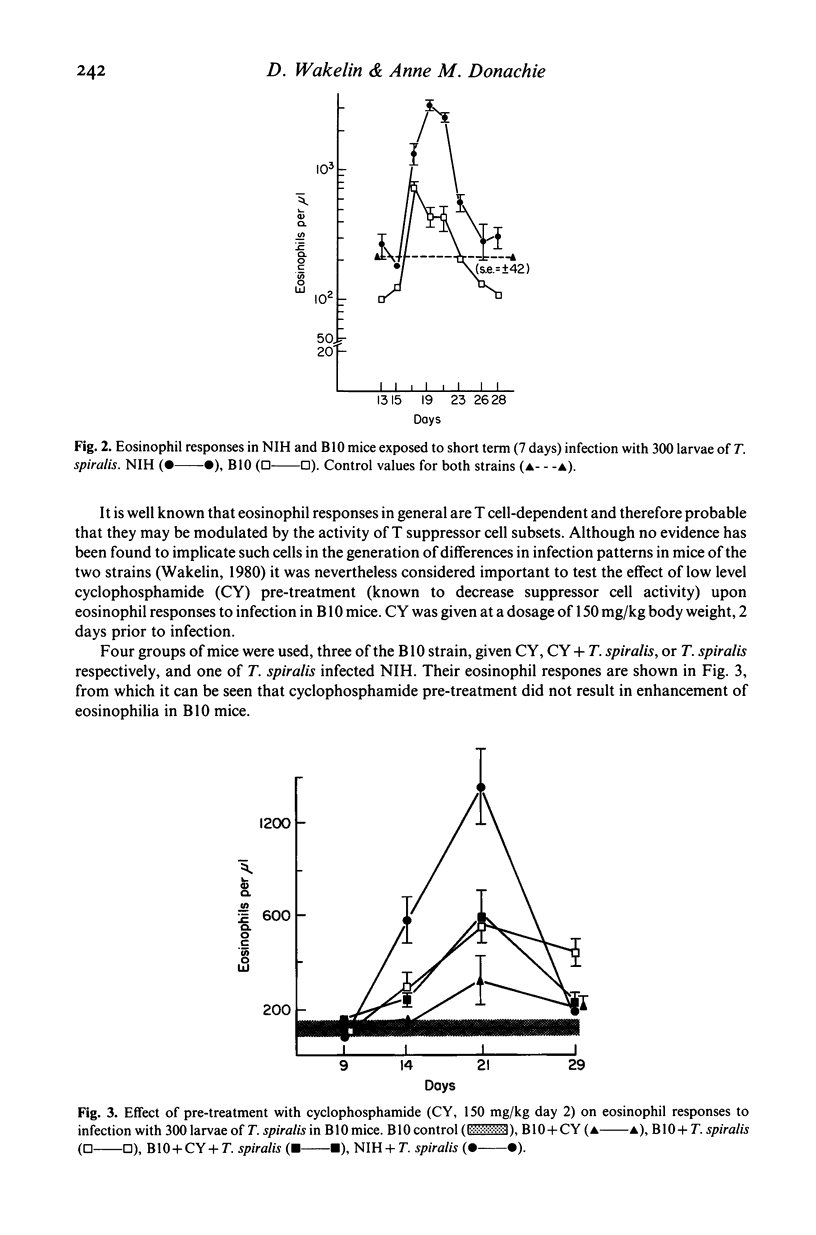
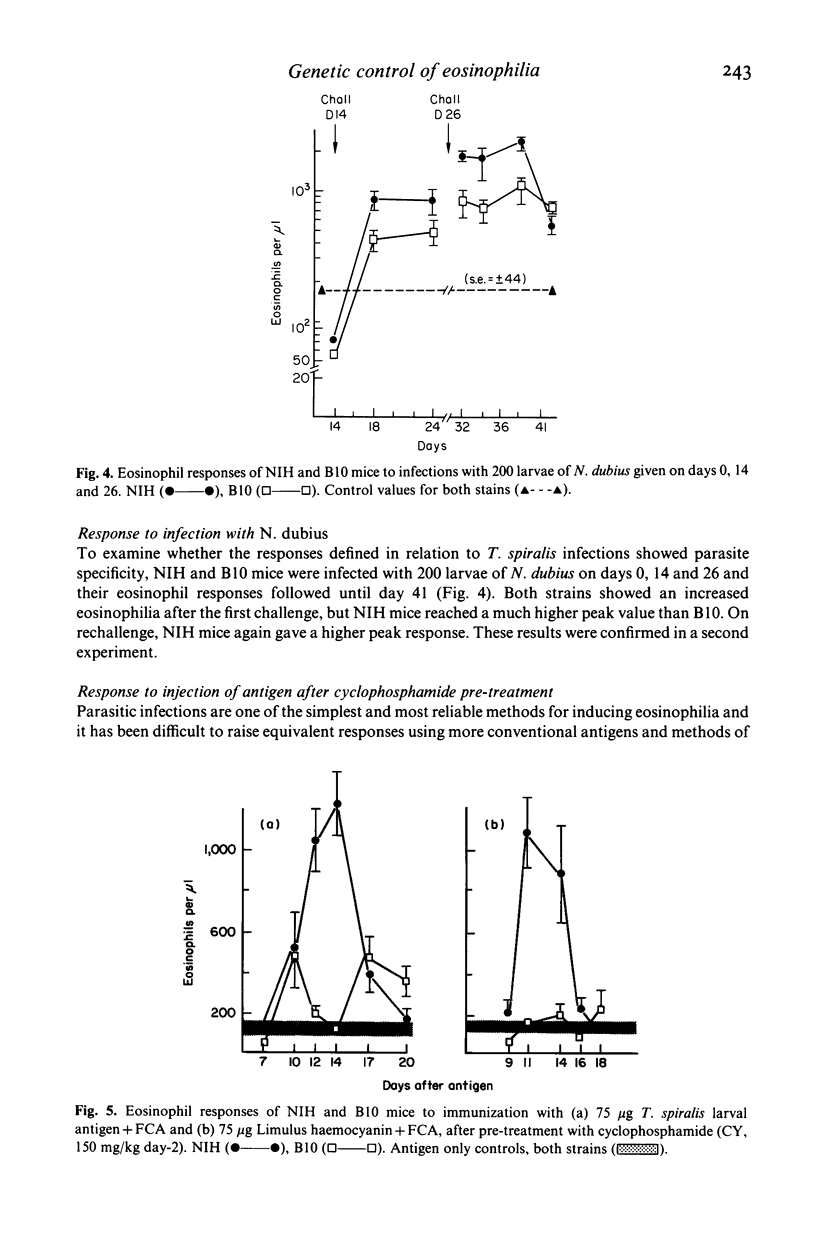
Strain variation in capacity to develop peripheral blood eosinophilia was observed in inbred NIH and C57BL/10 (B10) mice exposed to parasite antigens by infection or by parenteral injection in Freund's complete adjuvant. NIH mice were good responders, showing rapid development of high eosinophil counts, B10 mice were low responders. The difference in response phenotype was independent of the parasite used for infection (Trichinella spiralis or Nematospiroides dubius) and of the antigen used for injection (T. spiralis larval antigen or Limulus haemocyanin). Pre-treatment of T. spiralis infected mice with low doses of cyclophosphamide (150 mg/kg) or restriction of the duration of infection to 7 days by anthelmintic treatment did not enhance the response of B10 mice. Thus no evidence was found that the poor response phenotype of B10 during T. spiralis infection reflected any active suppressive mechanisms developed during the adult or muscle larval phases of infection. Demonstration that eosinophilia is induced primarily by the intestinal phase allows comparison with other parameters of the immune response induced by the adult worms, namely intestinal mastocytosis and worm expulsion. From this comparison it is concluded that the low eosinophil response phenotype of B10 mice may reflect a generalized deficiency in the response of bone marrow derived precursor cells to factors of T lymphocyte origin. The significance of genetically determined variation in eosinophil responsiveness is discussed in relation to the development of protective immune or pathological responses to parasite infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizadeh H., Wakelin D. Genetic factors controlling the intestinal mast cell response in mice infected with Trichinella spiralis. Clin Exp Immunol. 1982 Aug;49(2):331–337. [PMC free article] [PubMed] [Google Scholar]
- Bartelmez S. H., Dodge W. H., Bass D. A. Antigen-mediated release of eosinophil growth stimulating factor from Trichinella spiralis sensitized spleen cells: a comparison of T. spiralis stage-specific antigen preparations. Immunology. 1982 Apr;45(4):605–611. [PMC free article] [PubMed] [Google Scholar]
- Basten A., Boyer M. H., Beeson P. B. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970 Jun 1;131(6):1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant V. The life cycle of Nematospiroides dubius, Baylis, 1926 (Nematoda: Heligmosomidae). J Helminthol. 1973;47(3):263–268. doi: 10.1017/s0022149x00026535. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E. The eosinophil and its role in immunity to helminth infection. Curr Top Microbiol Immunol. 1977;77:127–168. doi: 10.1007/978-3-642-66740-4_5. [DOI] [PubMed] [Google Scholar]
- Claas F. H., Deelder A. M. H-2 linked immune response to murine experimental Schistosoma mansoni infections. J Immunogenet. 1979 Jun;6(3):167–175. doi: 10.1111/j.1744-313x.1979.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Despommier D., Weisbroth S., Fass C. Circulating eosinophils and trichinosis in the rat: the parasitic stage responsible for induction during infection. J Parasitol. 1974 Apr;60(2):280–284. [PubMed] [Google Scholar]
- Grove D. I., Mahmoud A. A., Warren K. S. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977 Mar 1;145(3):755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B. The role of the eosinophil. J Allergy Clin Immunol. 1979 Aug;64(2):90–104. doi: 10.1016/0091-6749(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Lee T. D., Grencis R. K., Wakelin D. Specific cross-immunity between Trichinella spiralis and Trichuris muris: immunization with heterologous infections and antigens and transfer of immunity with heterologous immune mesenteric lymph node cells. Parasitology. 1982 Apr;84(Pt 2):381–389. doi: 10.1017/s0031182000044929. [DOI] [PubMed] [Google Scholar]
- Ljungström I., Huldt G. Effect of experimental trichinosis on unrelated humoral and cell mediated immunity. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):131–141. doi: 10.1111/j.1699-0463.1977.tb03622.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Stone M. K., Kellermeyer R. W. Eosinophilopoietin. A circulating low molecular weight peptide-like substance which stimulates the production of eosinophils in mice. J Clin Invest. 1977 Sep;60(3):675–682. doi: 10.1172/JCI108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie B. M., Askenase P. W., Rose M. E. Basophils and eosinophils in three strains of rats and in athymic (nude) rats following infection with the nematodes Nippostrongylus brasiliensis or Trichinella spiralis. Immunology. 1980 Mar;39(3):385–389. [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A., Kruizinga W., Leenstra F. Trichinella spiralis infection in congenitally athymic (nude) mice. Parasitological, serological and haematological studies with observations on intestinal pathology. Immunology. 1977 Oct;33(4):581–587. [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Cypess R. H., Chervenick P. A. Specific release of neutrophillic- and eosinophilic-stimulating factors from sensitized lymphocytes. Blood. 1976 May;47(5):757–765. [PubMed] [Google Scholar]
- Spry C. J. Alterations in blood eosinophil morphology, binding capacity for complexed IgG and kinetics in patients with tropical (filarial) eosinophilia. Parasite Immunol. 1981 Spring;3(1):1–11. doi: 10.1111/j.1365-3024.1981.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Vadas M. A. Cyclophosphamide pretreatment induces eosinophilia to nonparasite antigens. J Immunol. 1981 Nov;127(5):2083–2086. [PubMed] [Google Scholar]
- Vadas M. A. Genetic control of eosinophilia in mice: gene(s) expressed in bone marrow-derived cells control high responsiveness. J Immunol. 1982 Feb;128(2):691–695. [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to Trichinella spiralis. Donor bone marrow cells determine responses to infection in mouse radiation chimaeras. Immunology. 1981 Aug;43(4):787–792. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to parasites: adoptive transfer of immunity between inbred strains of mice characterized by rapid and slow immune expulsion of Trichinella spiralis. Parasite Immunol. 1980 Winter;2(4):249–260. doi: 10.1111/j.1365-3024.1980.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Lloyd M. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology. 1976 Apr;72(2):173–182. doi: 10.1017/s0031182000048472. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Goetzl E. J. The regulatory and effector roles of eosinophils. Adv Immunol. 1979;27:339–371. doi: 10.1016/s0065-2776(08)60264-3. [DOI] [PubMed] [Google Scholar]


