Abstract
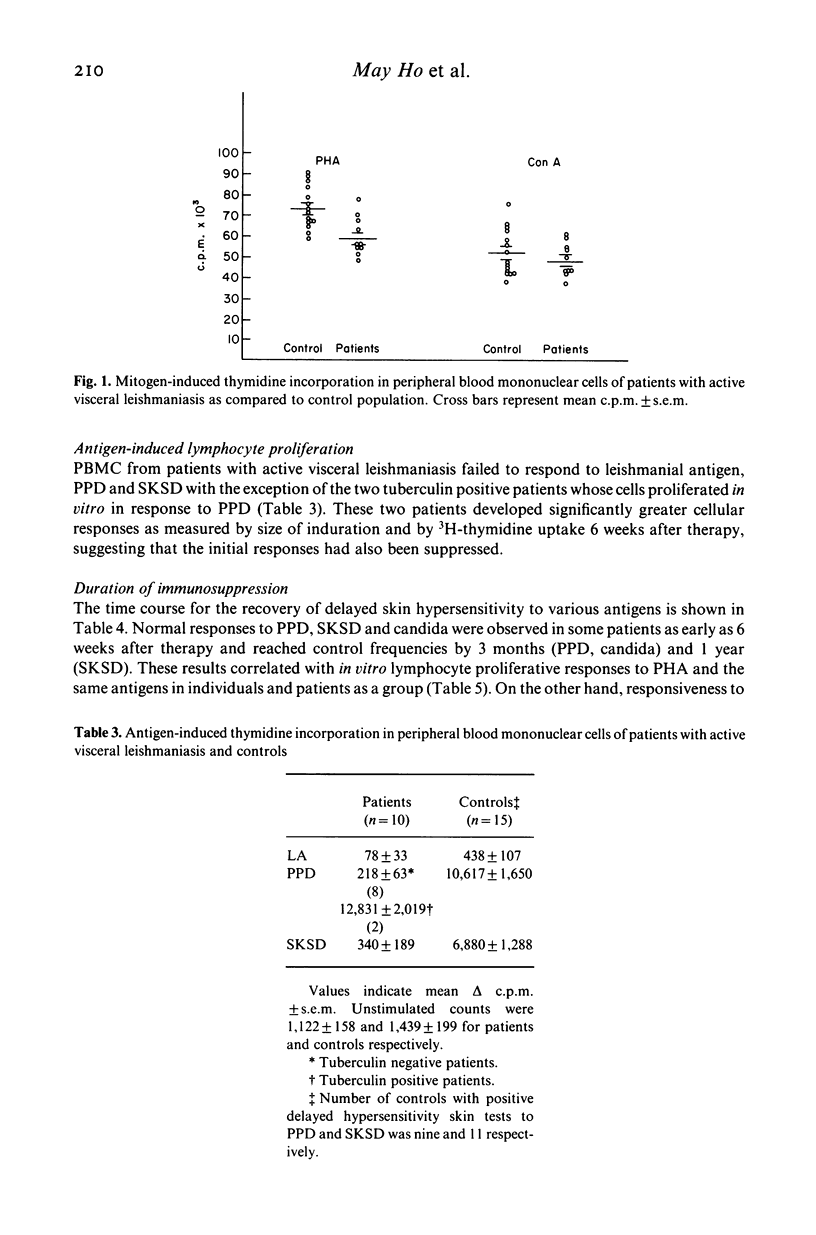
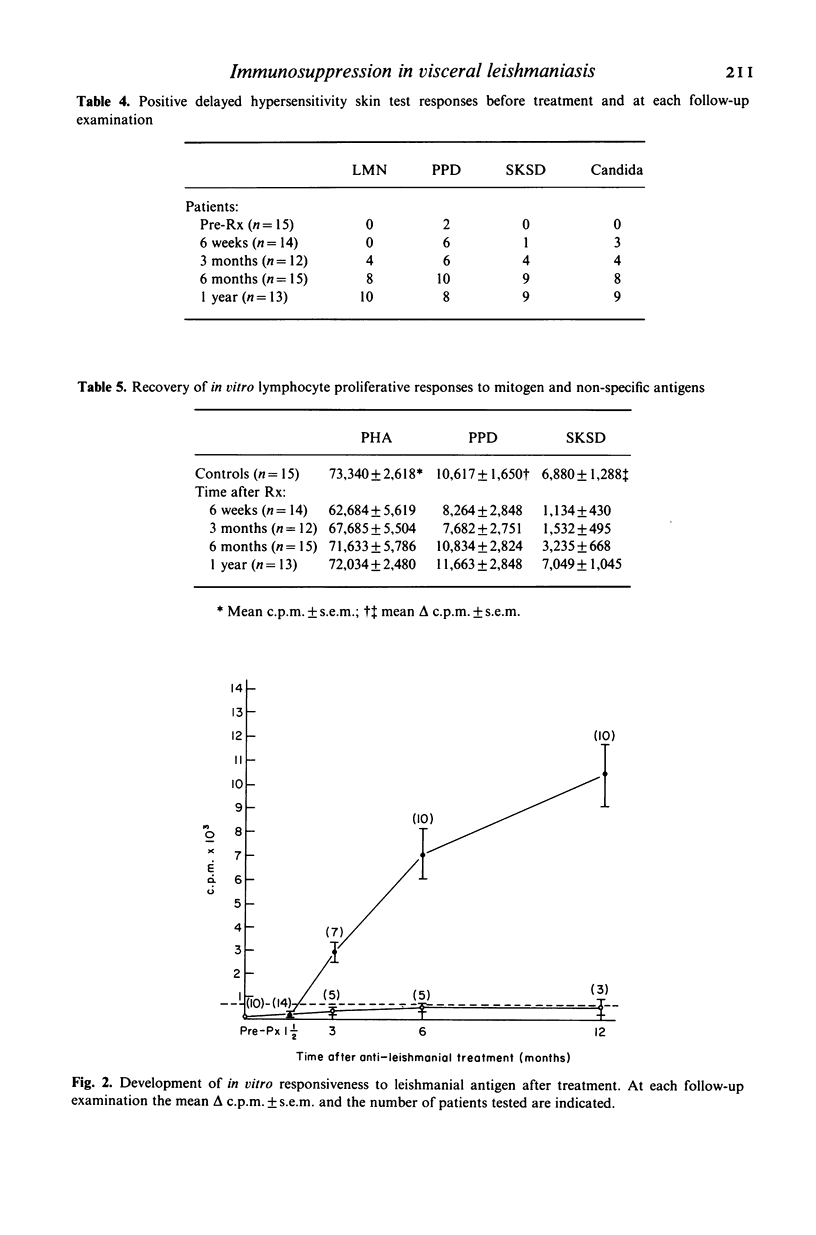
Cell-mediated immune responses were evaluated in 15 patients with active visceral leishmaniasis from Masinga location in eastern Kenya where the disease is endemic. Age and sex matched controls were selected from a village school in the same area. In vivo studies were carried out by skin testing with leishmanin, tuberculin, streptococcal and candida antigens. Lymphocyte blastogenic transformation to the mitogens phytohaemagglutinin (PHA) and concanavalin A (Con A) and the antigens purified protein derivative (PPD), streptokinase-streptodornase (SKSD) and leishmanial antigen (LA) was studied in vitro. The results showed that immunosuppression in visceral leishmaniasis in Kenya was both specific and non-specific. In the majority of patients there was complete anergy to all antigens in vivo and in vitro. The suppression of responses to mitogens was less marked. Recovery of non-specific responses preceded the development of specific immunity. In a small number of patients (23%) immune unresponsiveness to leishmanial antigens persisted 1 year after parasitological cure.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikat B. K., Pathania A. G., Sehgal S., Bhattacharya P. K., Dutta U., Pasricha N., Singh S., Parmar R. S., Sahaya S., Prasad L. S. Immunological response in Indian kala-azar. Indian J Med Res. 1979 Oct;70:583–591. [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Suen J. Y., Tranum B. L. Immunological skin testing and interpretation: a plea for uniformity. Cancer. 1979 Jun;43(6):2306–2314. doi: 10.1002/1097-0142(197906)43:6<2306::aid-cncr2820430621>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Beatty D. W., Dowdle E. B. Deficiency in kwashiorkor serum of factors required for optimal lymphocyte transformation in vitro. Clin Exp Immunol. 1979 Mar;35(3):433–442. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. I. The clinical and histological features of the disease. Trans R Soc Trop Med Hyg. 1969;63(6):708–737. doi: 10.1016/0035-9203(69)90116-3. [DOI] [PubMed] [Google Scholar]
- CAHILL K. M. LEISHMANIASIS IN THE SUDAN REPUBLIC. XXI. INFECTION IN AMERICAN PERSONNEL. Am J Trop Med Hyg. 1964 Nov;13:794–799. doi: 10.4269/ajtmh.1964.13.794. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Phytohaemagglutinin-induced lymphocyte transformation test in Indian kala-azar. Trans R Soc Trop Med Hyg. 1979;73(6):725–726. doi: 10.1016/0035-9203(79)90032-4. [DOI] [PubMed] [Google Scholar]
- Hepner Levy L., Mendes E. Impaired cell-mediated immunity in patients with kala-azar. Allergol Immunopathol (Madr) 1981 Mar-Apr;9(2):109–112. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANSON-BAHR P. E. East African kalazar with special reference to the pathology, prophylaxis and treatment. Trans R Soc Trop Med Hyg. 1959 Mar;53(2):123–137. doi: 10.1016/0035-9203(59)90060-4. [DOI] [PubMed] [Google Scholar]
- Musumeci S., Schilirò G., Li Volti S., Sciotto A. Lymphocyte changes in Mediterranean kala-azar. Trans R Soc Trop Med Hyg. 1981;75(2):304–305. doi: 10.1016/0035-9203(81)90342-4. [DOI] [PubMed] [Google Scholar]
- Petersen E. A., Neva F. A., Oster C. N., Bogaert Diaz H. Specific inhibition of lymphocyte-proliferation responses by adherent suppressor cells in diffuse cutaneous leishmaniasis. N Engl J Med. 1982 Feb 18;306(7):387–392. doi: 10.1056/NEJM198202183060702. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- Sokal J. E. Editorial: Measurement of delayed skin-test responses. N Engl J Med. 1975 Sep 4;293(10):501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- TUCKMAN E. Treatment of Chinese kala-azar with sodium antimony gluconate. J Trop Med Hyg. 1949 Oct;52(10):199–204. [PubMed] [Google Scholar]
- Veress B., Omer A., Satir A. A., El Hassan A. M. Morphology of the spleen and lymph nodes in fatal visceral leishmaniasis. Immunology. 1977 Nov;33(5):605–610. [PMC free article] [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Weinbaum F. I., Herrod H. R. Characterization of in vitro proliferative responses of human lymphocytes to leishmanial antigens. J Infect Dis. 1979 Aug;140(2):215–221. doi: 10.1093/infdis/140.2.215. [DOI] [PubMed] [Google Scholar]


