Abstract
The cellular organization of the thymus was investigated in 3- and 12-month-old NZB × SJL F1 hybrid (NS) mice. Age-dependent alterations were demonstrated which differed strikingly according to the sex of the animals. In female mice, marked abnormalities of the thymus developed during ageing. They consisted of a more or less pronounced hypertrophy accompanied by histological changes and modifications in the nature of the lymphocyte populations. Three types of qualitative changes were found at 12 months of age: (1) depletion of cortical thymocytes as evidenced by histology, by the evaluation of peanut-agglutinin (PNA) binding and by cell electrophoresis; (2) hyperplasia of the medullary lymphoid tissue, probably reflecting the expansion of a population of mature T lymphocytes. This was further suggested by a rise (up to 60%) in the frequency of lymphocytes lacking both PNA receptor and B cell markers, by an increased proportion (57%) of high electrophoretic mobility (EPM) lymphocytes and by an augmentation of in vitro reactivities to phytohaemagglutinin (PHA) and, although to a lesser extent, to concanavalin A (Con A). (3) The appearance of significant numbers of B lymphocytes (up to 20%) as assessed by surface immunoglobulin (sIg) and complement receptor (CR) detection which was accompanied by a vigorous responsiveness of thymus cells to lipopolysaccharide (LPS). None of these abnormalities was seen in the male mice. Instead, the thymus of NS males displayed a nearly normal age-related involution without major change in the proportions of its lymphocyte subpopulations. NS mice thus provide an interesting model of thymic disease influenced by sex-linked factors.
Full text
PDF


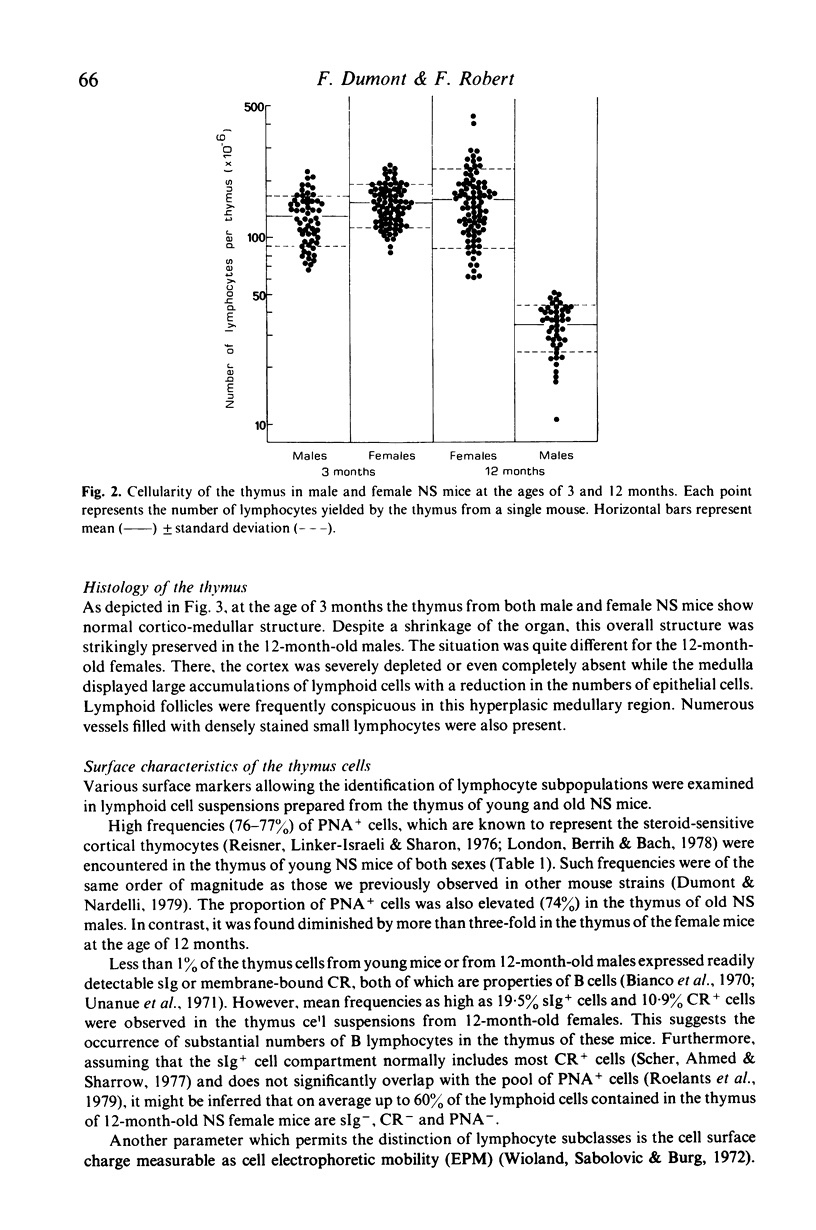
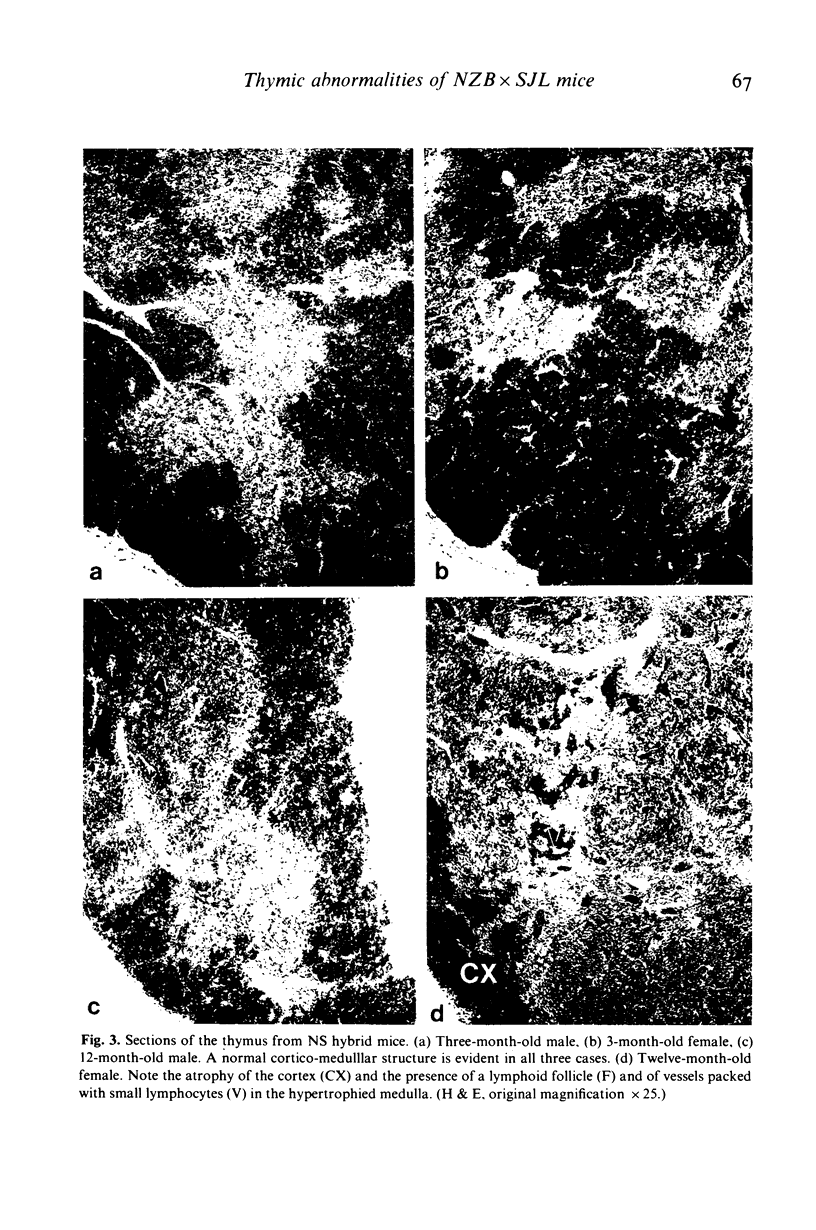
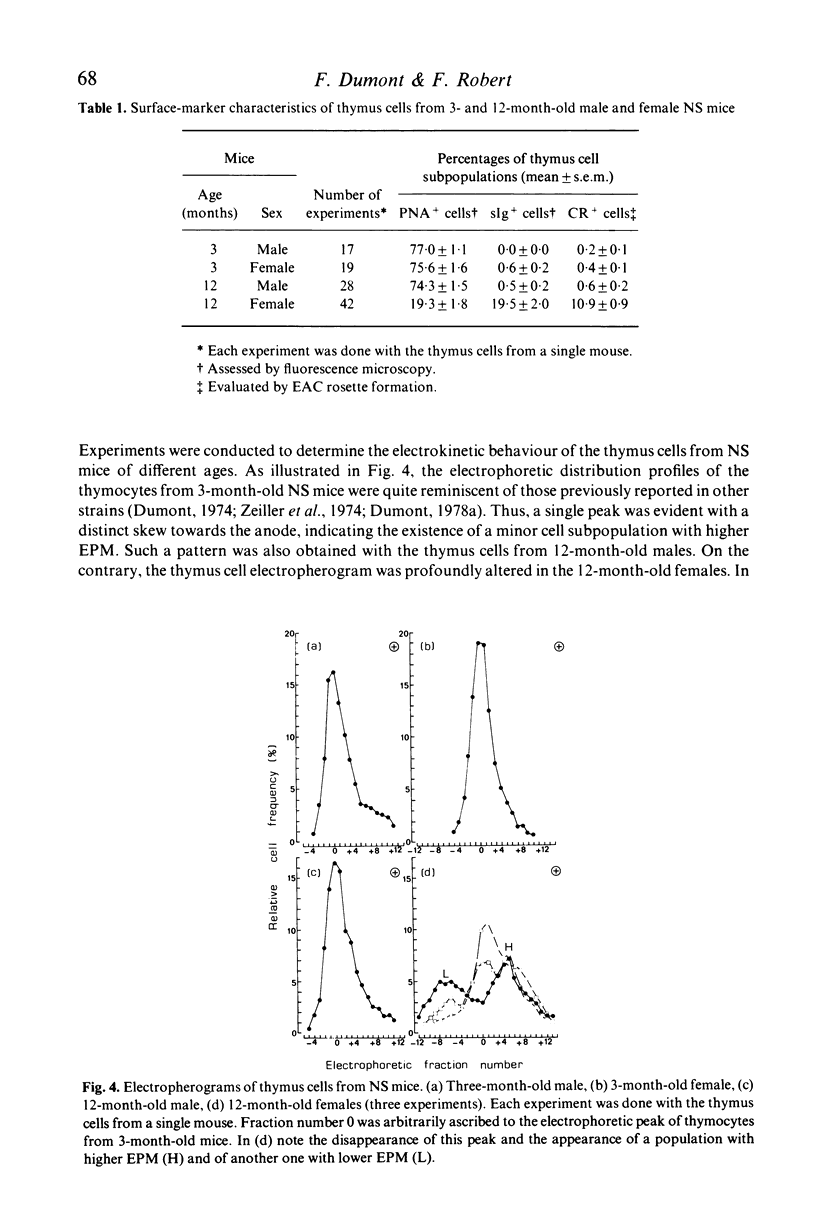




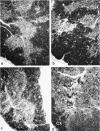
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNET F. M., HOLMES M. C. Immunological function of thymus and bursa of Fabricius. Thymus lesions in an auto-immune disease of mice. Nature. 1962 Apr 14;194:146–147. doi: 10.1038/194146a0. [DOI] [PubMed] [Google Scholar]
- Ben-Yaakov M., Haran-Ghera N. T & B lymphocytes in thymus of SJL/J mice. Nature. 1975 May 1;255(5503):64–66. doi: 10.1038/255064a0. [DOI] [PubMed] [Google Scholar]
- Dumont F. Physical subpopulations of mouse thymocytes: changes during regeneration subsequent to cortisone treatment. Immunology. 1978 May;34(5):841–852. [PMC free article] [PubMed] [Google Scholar]
- Dumont F., Robert F. Dose-related effect of hydrocortisone treatment on the electrokinetic properties and mitogen responsiveness of mouse thymocytes. Int Arch Allergy Appl Immunol. 1976;51(4):482–495. doi: 10.1159/000231622. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Gutman G. A., Weissman I. L. Lymphoid tissue architecture. Experimental analysis of the origin and distribution of T-cells and B-cells. Immunology. 1972 Oct;23(4):465–479. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- London J., Berrih S., Bach J. F. Peanut agglutinin. I. A new tool for studying T lymphocyte subpopulations. J Immunol. 1978 Aug;121(2):438–443. [PubMed] [Google Scholar]
- Melez K. A., Reeves J. P., Steinberg A. D. Modification of murine lupus by sex hormones. Ann Immunol (Paris) 1978 Jul-Sep;129 100(5):707–714. [PubMed] [Google Scholar]
- Murphy E. D. Transplantation behavior of Hodgkin's-like reticulum cell neoplasms of strain SJL-J mice and results of tumor reinoculation. J Natl Cancer Inst. 1969 May;42(5):797–807. [PubMed] [Google Scholar]
- Ozato K., Adler W. H., Ebert J. D. Synergism of bacterial lipopolysaccharides and concanavalin A in the activation of thymic lymphocytes. Cell Immunol. 1975 Jun;17(2):532–541. doi: 10.1016/s0008-8749(75)80057-8. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., London J., Mayor-Withey K. S., Serrano B. Peanut agglutinin. II. Characterization of the Thy-1, Tla and Ig phenotype of peanut agglutinin-positive cells in adult, embryonic and nude mice using double immunofluorescence. Eur J Immunol. 1979 Feb;9(2):139–145. doi: 10.1002/eji.1830090209. [DOI] [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978 Jun 1;147(6):1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I., Ahmed A., Sharrow S. O. Murine B lymphocyte heterogeneity: distribution of complement receptor-bearing and minor lymphocyte-stimulating B lymphocytes among cells with different densities of total surface Ig and IgM. J Immunol. 1977 Dec;119(6):1938–1942. [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Talal N., Steinberg A. D. The pathogenesis of autoimmunity in New Zealand black mice. Curr Top Microbiol Immunol. 1974;64(0):79–103. doi: 10.1007/978-3-642-65848-8_3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland M., Sabolovic D., Burg C. Electrophoretic mobilities of T and B cells. Nat New Biol. 1972 Jun 28;237(78):274–276. doi: 10.1038/newbio237274a0. [DOI] [PubMed] [Google Scholar]
- Zeiller K., Pascher G., Wagner G., Liebich H. G., Holzberg E., Hannig K. Distinct subpopulations of thymus-dependent lymphocytes. Tracing of the differentiation pathway of T cells by use of preparatively electrophoretically separated mouse lymphocytes. Immunology. 1974 May;26(5):995–1012. [PMC free article] [PubMed] [Google Scholar]